|
51
|
Wang T, Zhu Y, Chen L, Zhang W, Qi H, Shi X, Zhong M, Chen H, Li Q. ESRRG-PKM2 axis reprograms metabolism to suppress esophageal squamous carcinoma progression and enhance anti-PD-1 therapy efficacy. J Transl Med 2023; 21:605. [PMID: 37679788 PMCID: PMC10485992 DOI: 10.1186/s12967-023-04347-5] [Citation(s) in RCA: 6] [Impact Index Per Article: 3.0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 05/14/2023] [Accepted: 07/11/2023] [Indexed: 09/09/2023] Open
Abstract
BACKGROUND Glycolysis under normoxic conditions, known as the Warburg effect, confers a selective advantage for the survival and proliferation of many tumors. In this study, we investigated the role of estrogen-related receptor gamma (ESRRG) in metabolic reprogramming in esophageal squamous cell carcinoma (ESCC). METHODS Bioinformatics analysis indicated that ESRRG expression was decreased in ESCC tissue and associated with poor clinical outcomes. We also examined the effects of altered ESRRG expression on the proliferation and metabolic reprogramming of ESCC cells. We explored the impact of ESRRG on Pyruvate kinase M2 (PKM2) expression and malignant behavior in ESCC. RESULTS Our study revealed the inhibitory effects of ESRRG on the growth, tumorigenesis, and glycolysis activity of ESCC cells, which were mediated by the downregulation of PKM2 expression. We further demonstrated that ESRRG directly interacts with the PKM2 promoter to inhibit its activity in ESCC. Notably, the ESRRG-specific agonist, DY131, inhibited ESCC cell proliferation and glycolysis activity by modulating genes in the glycolysis pathway. Moreover, we verified that DY131 exhibits enhanced activity as an immune checkpoint inhibitor, considering the significance of the ESRRG-PKM2 axis in the lactate regulation of ESCC cells. CONCLUSION Our findings provide novel insights into the role of ESRRG-PKM2 signaling in regulating ESCC cell metabolism and immune checkpoint regulation. Additionally, we suggest that DY131 holds promise as a promising therapeutic agent for ESCC treatment.
Collapse
Affiliation(s)
- Tianxiao Wang
- Department of Pharmacy, Huashan Hospital, Fudan University, No.12 Urumqi Middle Road, Shanghai, 200040, China
| | - Yongjun Zhu
- Department of Cardio-Thoracic Surgery, Huashan Hospital, Fudan University, Shanghai, China
| | - Lu Chen
- Department of Pharmacy, Huashan Hospital, Fudan University, No.12 Urumqi Middle Road, Shanghai, 200040, China
| | - WenXin Zhang
- Department of Pharmacy, Huashan Hospital, Fudan University, No.12 Urumqi Middle Road, Shanghai, 200040, China
| | - Huijie Qi
- Department of Pharmacy, Huashan Hospital, Fudan University, No.12 Urumqi Middle Road, Shanghai, 200040, China
| | - Xiaojin Shi
- Department of Pharmacy, Huashan Hospital, Fudan University, No.12 Urumqi Middle Road, Shanghai, 200040, China
| | - Mingkang Zhong
- Department of Pharmacy, Huashan Hospital, Fudan University, No.12 Urumqi Middle Road, Shanghai, 200040, China
| | - Haifei Chen
- Department of Pharmacy, Huashan Hospital, Fudan University, No.12 Urumqi Middle Road, Shanghai, 200040, China.
| | - Qunyi Li
- Department of Pharmacy, Huashan Hospital, Fudan University, No.12 Urumqi Middle Road, Shanghai, 200040, China.
| |
Collapse
|
|
52
|
Aldayyeni H, Hjazi A, Shahab S, Gupta J, Alsaab HO, Motea YH, Alazbjee AAA, Romero-Parra RM, Obaid RF, Hussien BM, Hosseini-Fard SR. Functions, mechanisms, and clinical applications of lncRNA LINC00857 in cancer pathogenesis. Hum Cell 2023; 36:1656-1671. [PMID: 37378889 DOI: 10.1007/s13577-023-00936-0] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 04/11/2023] [Accepted: 06/12/2023] [Indexed: 06/29/2023]
Abstract
Emerging data indicated that long noncoding RNAs (lncRNAs) are crucial players in the biological processes via regulating epigenetics, transcription, and protein translation. A novel lncRNA, LINC00857, was indicated to upregulate in several types of cancer. In addition, LINC00857 was functionally related to the modulation of the cancer-linked behaviors, including invasion, migration, proliferation, epithelial-mesenchymal transition (EMT), cell cycle, and apoptosis. The importance of LINC00857 in cancer onset and development proposed that LINC00857 has major importance in the cancer progression and may be considered as a novel prognostic/diagnostic biomarker as well as a treatment target. Here, we retrospectively investigate the available progress in biomedical research investigating the functions of LINC00857 in cancer, focusing on finding the molecular mechanisms affecting various cancer-related behaviors and exploring its clinical applications.
Collapse
Affiliation(s)
| | - Ahmed Hjazi
- Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, Al-Kharj, 11942, Saudi Arabia
| | - Sana Shahab
- Department of Business Administration, College of Business Administration, Princess Nourah Bint Abdulrahman University, P.O. Box 84428, Riyadh, 11671, Saudi Arabia
| | - Jitendra Gupta
- Institute of Pharmaceutical Research, GLA University, Mathura, Uttar Pradesh, 281406, India
| | - Hashem O Alsaab
- Department of Pharmaceutics and Pharmaceutical Technology, Taif University, Taif, 21944, Saudi Arabia
| | | | | | | | - Rasha Fadhel Obaid
- Department of Biomedical Engineering, Al-Mustaqbal University College, Babylon, Iraq
| | - Beneen M Hussien
- Medical Laboratory Technology Department, College of Medical Technology, The Islamic University, Najaf, Iraq
| | - Seyed Reza Hosseini-Fard
- Department of Biochemistry, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
| |
Collapse
|
|
53
|
Zhou Y, Xue X, Luo J, Li P, Xiao Z, Zhang W, Zhou J, Li P, Zhao J, Ge H, Tian Z, Zhao X. Circular RNA circ-FIRRE interacts with HNRNPC to promote esophageal squamous cell carcinoma progression by stabilizing GLI2 mRNA. Cancer Sci 2023; 114:3608-3622. [PMID: 37417427 PMCID: PMC10475760 DOI: 10.1111/cas.15899] [Citation(s) in RCA: 16] [Impact Index Per Article: 8.0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 02/05/2023] [Revised: 05/29/2023] [Accepted: 06/18/2023] [Indexed: 07/08/2023] Open
Abstract
Increasing evidence has shown that circular RNAs (circRNAs) interact with RNA-binding proteins (RBPs) and promote cancer progression. However, the function and mechanism of the circRNA/RBP complex in esophageal squamous cell carcinoma (ESCC) are still largely unknown. Herein, we first characterized a novel oncogenic circRNA, circ-FIRRE, by RNA sequencing (Ribo-free) profiling of ESCC samples. Furthermore, we observed marked circ-FIRRE overexpression in ESCC patients with high TNM stage and poor overall survival. Mechanistic studies indicated that circ-FIRRE, as a platform, interacts with the heterogeneous nuclear ribonucleoprotein C (HNRNPC) protein to stabilize GLI2 mRNA by directly binding to its 3'-UTR in the cytoplasm, thereby resulting in elevated GLI2 protein expression and subsequent transcription of its target genes MYC, CCNE1, and CCNE2, ultimately contributing to ESCC progression. Moreover, HNRNPC overexpression in circ-FIRRE knockdown cells notably abolished circ-FIRRE knockdown-mediated Hedgehog pathway inhibition and ESCC progression impairment in vitro and in vivo. Clinical specimen results showed that circ-FIRRE and HNRNPC expression was positively correlated with GLI2 expression, which reveals the clear significance of the circ-FIRRE/HNRNPC-GLI2 axis in ESCC. In summary, our results indicate that circ-FIRRE could serve as a valuable biomarker and potential therapeutic target for ESCC and highlight a novel mechanism of the circ-FIRRE/HNRNPC complex in ESCC progression regulation.
Collapse
Affiliation(s)
- Yongjia Zhou
- Department of Thoracic SurgeryThe Second Hospital of Shandong UniversityJinanChina
| | - Xia Xue
- Department of PharmacyThe Second Hospital of Shandong UniversityJinanChina
| | - Junwen Luo
- Department of Thoracic SurgeryThe Second Hospital of Shandong UniversityJinanChina
| | - Peiwei Li
- Institute of Medical SciencesThe Second Hospital of Shandong UniversityJinanChina
| | - Zhaohua Xiao
- Department of Thoracic SurgeryThe Second Hospital of Shandong UniversityJinanChina
| | - Wenhao Zhang
- Department of Thoracic SurgeryThe Second Hospital of Shandong UniversityJinanChina
| | - Jie Zhou
- Department of Thoracic SurgeryThe Second Hospital of Shandong UniversityJinanChina
| | - Peichao Li
- Department of Thoracic SurgeryThe Second Hospital of Shandong UniversityJinanChina
| | - Jiangfeng Zhao
- Department of Thoracic SurgeryThe Second Hospital of Shandong UniversityJinanChina
| | - Haibo Ge
- Department of Thoracic SurgeryThe Second Hospital of Shandong UniversityJinanChina
| | - Zhongxian Tian
- Department of Thoracic SurgeryThe Second Hospital of Shandong UniversityJinanChina
- Key Laboratory of Thoracic Cancer in Universities of ShandongThe Second Hospital of Shandong UniversityJinanChina
| | - Xiaogang Zhao
- Department of Thoracic SurgeryThe Second Hospital of Shandong UniversityJinanChina
- Key Laboratory of Thoracic Cancer in Universities of ShandongThe Second Hospital of Shandong UniversityJinanChina
| |
Collapse
|
|
54
|
Knapp K, Verchio V, Coburn-Flynn O, Li Y, Xiong Z, Morrison JC, Shersher DD, Spitz F, Chen X. Exploring cell competition for the prevention and therapy of esophageal squamous cell carcinoma. Biochem Pharmacol 2023; 214:115639. [PMID: 37290594 PMCID: PMC10528900 DOI: 10.1016/j.bcp.2023.115639] [Citation(s) in RCA: 1] [Impact Index Per Article: 0.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 04/03/2023] [Revised: 06/01/2023] [Accepted: 06/02/2023] [Indexed: 06/10/2023]
Abstract
Esophageal squamous cell carcinoma (ESCC) is characterized by the development of cancer in the esophageal squamous epithelium through a step-by-step accumulation of genetic, epigenetic, and histopathological alterations. Recent studies have demonstrated that cancer-associated gene mutations exist in histologically normal or precancerous clones of the human esophageal epithelium. However, only a small proportion of such mutant clones will develop ESCC, and most ESCC patients develop only one cancer. This suggests that most of these mutant clones are kept in a histologically normal state by neighboring cells with higher competitive fitness. When some of the mutant cells evade cell competition, they become "super-competitors" and develop into clinical cancer. It is known that human ESCC is composed of a heterogeneous population of cancer cells that interact with and influence their environment and neighbors. During cancer therapy, these cancer cells not only respond to therapeutic agents but also compete with each other. Therefore, competition between ESCC cells within the same ESCC tumor is a constantly dynamic process. However, it remains challenging to fine-tune the competitive fitness of various clones for therapeutic benefits. In this review, we will explore the role of cell competition in carcinogenesis, cancer prevention, and therapy, using NRF2, NOTCH pathway, and TP53 as examples. We believe that cell competition is a research area with promising targets for clinical translation. Manipulating cell competition may help improve the prevention and therapy of ESCC.
Collapse
Affiliation(s)
- Kristen Knapp
- Department of Surgery, Cooper University Hospital, Camden, NJ 08103, USA
| | - Vincent Verchio
- Department of Surgery, Cooper University Hospital, Camden, NJ 08103, USA
| | | | - Yahui Li
- Coriell Institute for Medical Research, Camden, NJ 08103, USA
| | - Zhaohui Xiong
- Coriell Institute for Medical Research, Camden, NJ 08103, USA
| | - Jamin C Morrison
- MD Anderson Cancer Center at Cooper, Camden, NJ 08103, USA; Cooper Medical School of Rowan University, Camden, NJ 08103, USA
| | - David D Shersher
- Department of Surgery, Cooper University Hospital, Camden, NJ 08103, USA; MD Anderson Cancer Center at Cooper, Camden, NJ 08103, USA; Cooper Medical School of Rowan University, Camden, NJ 08103, USA
| | - Francis Spitz
- Department of Surgery, Cooper University Hospital, Camden, NJ 08103, USA; MD Anderson Cancer Center at Cooper, Camden, NJ 08103, USA; Cooper Medical School of Rowan University, Camden, NJ 08103, USA
| | - Xiaoxin Chen
- Department of Surgery, Cooper University Hospital, Camden, NJ 08103, USA; Coriell Institute for Medical Research, Camden, NJ 08103, USA; MD Anderson Cancer Center at Cooper, Camden, NJ 08103, USA; Cooper Medical School of Rowan University, Camden, NJ 08103, USA.
| |
Collapse
|
|
55
|
Dai X, Tao L, Wang J, Wu W, Bian W, Dai X, Chen S. Efficacy and safety of irinotecan combined with raltitrexed or irinotecan monotherapy for salvage chemotherapy of esophageal squamous cell cancer: A prospective, open label, randomized phase II study. Cancer Med 2023; 12:16108-16118. [PMID: 37325938 PMCID: PMC10469638 DOI: 10.1002/cam4.6264] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/25/2023] [Revised: 05/29/2023] [Accepted: 06/06/2023] [Indexed: 06/17/2023] Open
Abstract
BACKGROUND Esophageal squamous cell cancer (ESCC) accounts for approximately 90% of esophageal cancer cases in China. There are no standard regimens for second or third-line chemotherapy of metastatic squamous esophageal cancer. The objective of this study was to investigate the security and effectiveness of irinotecan combined with raltitrexed or irinotecan monotherapy for salvage chemotherapy of ESCC. METHODS One hundred and twenty-eight patients with metastatic ESCC confirmed by histopathology were enrolled into this study. These patients had failure of the first-line chemotherapy combination of fluorouracil or platinum or paclitaxel and had not undergone chemotherapy with irinotecan or raltitrexed previously. Patients were randomly divided into irinotecan combined with raltitrexed group (experiment group) and irinotecan monotherapy group (control group). Overall survival (OS) and progression-free survival (PFS) were the primary endpoint. RESULTS In the control group, the median PFS (mPFS) and median OS (mOS) of patients were 3.37 and 5.3 months. In the experiment group, mPFS and mOS were 3.91 and 7.0 months. There was statistical significance of PFS and OS between two groups (PFS P = 0.002, OS P = 0.01). In subgroup analysis, in the second-line treatment, the mPFS of control and experiment group, was 3.90 and 4.60 months, mOS was 6.95 and 8.5 months, which was statistically significant differences between the two groups. (PFS P = 0.001, OS P = 0.005), In the third-line and beyond treatment, mPFS of control and experiment group was 2.80 and 3.19 months, mOS were 4.5 and 4.8 months. But there was no significant difference of PFS or OS between the two groups (PFS P = 0.19, OS P = 0.31). There was no statistical significance of toxicity side effects between two groups. CONCLUSIONS The PFS and OS of irinotecan plus raltitrexed may be better than that of irinotecan monotherapy, especially in second line treatment, which should be confirmed with a phase III study including much more patients.
Collapse
Affiliation(s)
- Xichao Dai
- Department of OncologyFirst people's Hospital of YanchengYanchengChina
- Yancheng Clinical College of Xuzhou Medical UniversityXuzhouChina
- The Fourth Affiliated Hospital of Nantong UniversityNantongChina
| | - Leilei Tao
- Department of OncologyFirst people's Hospital of YanchengYanchengChina
- Yancheng Clinical College of Xuzhou Medical UniversityXuzhouChina
- The Fourth Affiliated Hospital of Nantong UniversityNantongChina
| | - Jinqiu Wang
- Yancheng Dafeng People's HospitalYanchengChina
| | - Wenjuan Wu
- Department of OncologyNorthern Jiangsu People's Hospital, Clinical Medical College of Yangzhou UniversityYangzhouChina
| | - Weigang Bian
- Department of OncologyFirst people's Hospital of YanchengYanchengChina
- Yancheng Clinical College of Xuzhou Medical UniversityXuzhouChina
- The Fourth Affiliated Hospital of Nantong UniversityNantongChina
| | - Xichun Dai
- Department of OncologyHongze People's Hospital of Huai'an CityChina
| | - Surong Chen
- Department of OncologyFirst people's Hospital of YanchengYanchengChina
- Yancheng Clinical College of Xuzhou Medical UniversityXuzhouChina
- The Fourth Affiliated Hospital of Nantong UniversityNantongChina
| |
Collapse
|
|
56
|
Tang Q, Li Q, Shi L, Liu W, Li B, Jin Y. Multifunctional DNA nanoprobe for tumor-targeted synergistic therapy by integrating chemodynamic therapy with gene silencing. NANOSCALE HORIZONS 2023; 8:1106-1112. [PMID: 37317707 DOI: 10.1039/d2nh00575a] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [MESH Headings] [Track Full Text] [Subscribe] [Scholar Register] [Indexed: 06/16/2023]
Abstract
Due to the high complexity, diversity and heterogeneity of tumor occurrence and development, multi-mode synergistic therapy is more effective than single treatment modes to improve the antitumor efficacy. Also, multifunctional probes are crucial to realize synergistic therapy. Herein, a multifunctional DNA tetrahedron nanoprobe was ingeniously designed to simultaneously achieve chemodynamic therapy (CDT) and gene silencing for synergistic antitumor. The multifunctional DNA tetrahedron nanoprobe, DNA tetrahedron-silver nanocluster-antagomir-21 (D-sgc8-DTNS-AgNCs-Anta-21), integrated a CDT reagent (DNA-AgNCs) and miRNA-21 inhibitor (Anta-21) with a specific recognition probe (aptamer). After targeted entry in cancer cells, D-sgc8-DTNS-AgNCs-Anta-21 silenced endogenous miRNA-21 by Anta-21 and produced highly toxic ˙OH by reacting with H2O2, which induced apoptosis in the tumor cells. The targeted recognition of aptamers led to the concentration-dependent death of HeLa cells. On the contrary, the cell survival rate of normal cells was basically unaffected with an increase in the concentration of D-sgc8-DTNS-AgNCs-Anta-21. Therefore, the diverse functions, biocompatibility and programmability of DNA provide a useful and easy way to assemble multifunctional probes for synergistic therapy.
Collapse
Affiliation(s)
- Qiaorong Tang
- Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China.
| | - Qianqian Li
- Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China.
| | - Lu Shi
- Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China.
| | - Wei Liu
- Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China.
| | - Baoxin Li
- Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China.
| | - Yan Jin
- Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China.
| |
Collapse
|
|
57
|
Mangalaparthi KK, Patel K, Khan AA, Nair B, Kumar RV, Prasad TSK, Sidransky D, Chatterjee A, Pandey A, Gowda H. Molecular Characterization of Esophageal Squamous Cell Carcinoma Using Quantitative Proteomics. Cancers (Basel) 2023; 15:3302. [PMID: 37444412 DOI: 10.3390/cancers15133302] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 02/15/2023] [Revised: 04/26/2023] [Accepted: 05/03/2023] [Indexed: 07/15/2023] Open
Abstract
Esophageal squamous cell carcinoma (ESCC) is a heterogeneous cancer associated with a poor prognosis in advanced stages. In India, it is the sixth most common cause of cancer-related mortality. In this study, we employed high-resolution mass spectrometry-based quantitative proteomics to characterize the differential protein expression pattern associated with ESCC. We identified several differentially expressed proteins including PDPN, TOP2A, POSTN and MMP2 that were overexpressed in ESCC. In addition, we identified downregulation of esophagus tissue-enriched proteins such as SLURP1, PADI1, CSTA, small proline-rich proteins such as SPRR3, SPRR2A, SPRR1A, KRT4, and KRT13, involved in squamous cell differentiation. We identified several overexpressed proteins mapped to the 3q24-29 chromosomal region, aligning with CNV alterations in this region reported in several published studies. Among these, we identified overexpression of SOX2, TP63, IGF2BP2 and RNF13 that are encoded by genes in the 3q26 region. Functional enrichment analysis revealed proteins involved in cell cycle pathways, DNA replication, spliceosome, and DNA repair pathways. We identified the overexpression of multiple proteins that play a major role in alleviating ER stress, including SYVN1 and SEL1L. The SYVN1/SEL1L complex is an essential part of the ER quality control machinery clearing misfolded proteins from the ER. SYVN1 is an E3 ubiquitin ligase that ubiquitinates ER-resident proteins. Interestingly, there are also other non-canonical substrates of SYVN1 which are known to play a crucial role in tumor progression. Thus, SYVN1 could be a potential therapeutic target in ESCC.
Collapse
Affiliation(s)
- Kiran K Mangalaparthi
- Institute of Bioinformatics, International Technology Park, Bangalore 560066, India
- Amrita School of Biotechnology, Amrita Vishwa Vidyapeetham, Kollam 691001, India
- Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN 55905, USA
| | - Krishna Patel
- Institute of Bioinformatics, International Technology Park, Bangalore 560066, India
- Amrita School of Biotechnology, Amrita Vishwa Vidyapeetham, Kollam 691001, India
| | - Aafaque Ahmad Khan
- Institute of Bioinformatics, International Technology Park, Bangalore 560066, India
| | - Bipin Nair
- Amrita School of Biotechnology, Amrita Vishwa Vidyapeetham, Kollam 691001, India
| | - Rekha V Kumar
- Department of Pathology, Kidwai Memorial Institute of Oncology, Bangalore 560066, India
| | - Thottethodi Subrahmanya Keshav Prasad
- Institute of Bioinformatics, International Technology Park, Bangalore 560066, India
- Amrita School of Biotechnology, Amrita Vishwa Vidyapeetham, Kollam 691001, India
- Center for Systems Biology and Molecular Medicine, Yenepoya Research Centre, Yenepoya (Deemed to be University), Mangalore 575018, India
| | - David Sidransky
- Department of Oncology, The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
- Department of Otolaryngology and Head & Neck Surgery, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA
| | - Aditi Chatterjee
- Institute of Bioinformatics, International Technology Park, Bangalore 560066, India
- Amrita School of Biotechnology, Amrita Vishwa Vidyapeetham, Kollam 691001, India
- Manipal Academy of Higher Education, Manipal 576104, India
| | - Akhilesh Pandey
- Institute of Bioinformatics, International Technology Park, Bangalore 560066, India
- Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN 55905, USA
- Manipal Academy of Higher Education, Manipal 576104, India
- Center for Individualized Medicine, Mayo Clinic, Rochester, MN 55905, USA
- Center for Molecular Medicine, National Institute of Mental Health and Neurosciences, Hosur Road, Bangalore 560029, India
| | - Harsha Gowda
- Institute of Bioinformatics, International Technology Park, Bangalore 560066, India
- Amrita School of Biotechnology, Amrita Vishwa Vidyapeetham, Kollam 691001, India
- Manipal Academy of Higher Education, Manipal 576104, India
| |
Collapse
|
|
58
|
Xu N, Li LS, Li H, Zhang LH, Zhang N, Wang PJ, Cheng YX, Xiang JY, Linghu EQ, Chai NL. SGK3 overexpression correlates with a poor prognosis in endoscopically resected superficial esophageal squamous cell neoplasia: A long-term study. World J Gastroenterol 2023; 29:3658-3667. [PMID: 37398883 PMCID: PMC10311610 DOI: 10.3748/wjg.v29.i23.3658] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Download PDF] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 02/24/2023] [Revised: 03/20/2023] [Accepted: 05/23/2023] [Indexed: 06/16/2023] Open
Abstract
BACKGROUND The expression status of serum and glucocorticoid-induced protein kinase 3 (SGK3) in superficial esophageal squamous cell neoplasia (ESCN) remains unknown. AIM To evaluate the SGK3 overexpression rate in ESCN and its influence on the prognosis and outcomes of patients with endoscopic resection. METHODS A total of 92 patients who had undergone endoscopic resection for ESCN with more than 8 years of follow-up were enrolled. Immunohistochemistry was used to evaluate SGK3 expression. RESULTS SGK3 was overexpressed in 55 (59.8%) patients with ESCN. SGK3 overexpression showed a significant correlation with death (P = 0.031). Overall survival and disease-free survival rates were higher in the normal SGK3 expression group than in the SGK3 overexpression group (P = 0.013 and P = 0.004, respectively). Cox regression analysis models demonstrated that SGK3 overexpression was an independent predictor of poor prognosis in ESCN patients (hazard ratio 4.729; 95% confidence interval: 1.042-21.458). CONCLUSION SGK3 overexpression was detected in the majority of patients with endoscopically resected ESCN and was significantly associated with shortened survival. Thus, it might be a new prognostic factor for ESCN.
Collapse
Affiliation(s)
- Ning Xu
- Senior Department of Gastroenterology, The First Medical Center of PLA General Hospital, Beijing 100853, China
| | - Long-Song Li
- Senior Department of Gastroenterology, The First Medical Center of PLA General Hospital, Beijing 100853, China
| | - Hui Li
- Department of Gastroenterology, Air Force Medical Center, Beijing 100142, China
| | - Li-Hua Zhang
- Department of Pathology, The Fourth Medical Center of PLA General Hospital, Beijing 100142, China
| | - Nan Zhang
- Senior Department of Gastroenterology, The First Medical Center of PLA General Hospital, Beijing 100853, China
| | - Peng-Ju Wang
- Senior Department of Gastroenterology, The First Medical Center of PLA General Hospital, Beijing 100853, China
| | - Ya-Xuan Cheng
- Senior Department of Gastroenterology, The First Medical Center of PLA General Hospital, Beijing 100853, China
| | - Jing-Yuan Xiang
- Senior Department of Gastroenterology, The First Medical Center of PLA General Hospital, Beijing 100853, China
| | - En-Qiang Linghu
- Senior Department of Gastroenterology, The First Medical Center of PLA General Hospital, Beijing 100853, China
| | - Ning-Li Chai
- Senior Department of Gastroenterology, The First Medical Center of PLA General Hospital, Beijing 100853, China
| |
Collapse
|
|
59
|
Zhao Y, Li Y, Zhu R, Feng R, Cui H, Yu X, Huang F, Zhang R, Chen X, Li L, Chen Y, Liu Y, Wang J, Du G, Liu Z. RPS15 interacted with IGF2BP1 to promote esophageal squamous cell carcinoma development via recognizing m 6A modification. Signal Transduct Target Ther 2023; 8:224. [PMID: 37264021 DOI: 10.1038/s41392-023-01428-1] [Citation(s) in RCA: 23] [Impact Index Per Article: 11.5] [Reference Citation Analysis] [Abstract] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/17/2022] [Revised: 02/23/2023] [Accepted: 03/24/2023] [Indexed: 06/03/2023] Open
Abstract
Increased rates of ribosome biogenesis have been recognized as hallmarks of many cancers and are associated with poor prognosis. Using a CRISPR synergistic activation mediator (SAM) system library targeting 89 ribosomal proteins (RPs) to screen for the most oncogenic functional RPs in human esophageal squamous cell carcinoma (ESCC), we found that high expression of RPS15 correlates with malignant phenotype and poor prognosis of ESCC. Gain and loss of function models revealed that RPS15 promotes ESCC cell metastasis and proliferation, both in vitro and in vivo. Mechanistic investigations demonstrated that RPS15 interacts with the K homology domain of insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1), which recognizes and directly binds the 3'-UTR of MKK6 and MAPK14 mRNA in an m6A-dependent manner, and promotes translation of core p38 MAPK pathway proteins. By combining targeted drug virtual screening and functional assays, we found that folic acid showed a therapeutic effect on ESCC by targeting RPS15, which was augmented by the combination with cisplatin. Inhibition of RPS15 by folic acid, IGF2BP1 ablation, or SB203580 treatment were able to suppress ESCC metastasis and proliferation via the p38 MAPK signaling pathway. Thus, RPS15 promotes ESCC progression via the p38 MAPK pathway and RPS15 inhibitors may serve as potential anti-ESCC drugs.
Collapse
Affiliation(s)
- Yahui Zhao
- State Key Laboratory of Molecular Oncology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Yang Li
- State Key Laboratory of Molecular Oncology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Rui Zhu
- State Key Laboratory of Molecular Oncology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Riyue Feng
- State Key Laboratory of Molecular Oncology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Heyang Cui
- Department of Oncology, Cancer Institute, Peking University Shenzhen Hospital, Shenzhen Peking University-Hong Kong University of Science and Technology (PKU-HKUST) Medical Center, Shenzhen, 518035, China
| | - Xiao Yu
- State Key Laboratory of Molecular Oncology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Furong Huang
- State Key Laboratory of Molecular Oncology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Ruixiang Zhang
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100021, China
| | - Xiankai Chen
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100021, China
| | - Lei Li
- State Key Laboratory of Molecular Oncology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
- Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, 518116, China
| | - Yinghui Chen
- School of Life Sciences, Tsinghua University, Beijing, 100084, China
| | - Yuhao Liu
- State Key Laboratory of Molecular Oncology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
- Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, 518116, China
| | - Jinhua Wang
- Key Laboratory of Drug Target Research and Drug Screen, Institute of Materia Medica, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, 100050, China
| | - Guanhua Du
- Key Laboratory of Drug Target Research and Drug Screen, Institute of Materia Medica, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, 100050, China
| | - Zhihua Liu
- State Key Laboratory of Molecular Oncology, National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China.
| |
Collapse
|
|
60
|
Li L, Zhu R, Zhou H, Cui C, Yu X, Liu Y, Yin Y, Li Y, Feng R, Katz JP, Zhao Y, Zhang Y, Zhang L, Liu Z. All-Trans Retinoic Acid Promotes a Tumor Suppressive OTUD6B-β-TrCP-SNAIL Axis in Esophageal Squamous Cell Carcinoma and Enhances Immunotherapy. ADVANCED SCIENCE (WEINHEIM, BADEN-WURTTEMBERG, GERMANY) 2023; 10:e2207458. [PMID: 37038094 PMCID: PMC10238178 DOI: 10.1002/advs.202207458] [Citation(s) in RCA: 1] [Impact Index Per Article: 0.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Subscribe] [Scholar Register] [Received: 12/15/2022] [Revised: 03/02/2023] [Indexed: 06/04/2023]
Abstract
β-TrCP is an E3 ubiquitin ligase that plays important roles in multiple human cancers including esophageal squamous cell carcinoma (ESCC). Analysis of ESCC patient samples reveal that only protein level but not transcript level of β-TrCP associated with patient prognosis, suggesting regulators of β-TrCP protein stability play an essential role in ESCC progression and may be novel targets to develop ESCC therapies. Although β-TrCP stability is known to be mediated by the ubiquitin-proteasome system, it is unclear which enzymes play a major role to determine β-TrCP stability in the context of ESCC. In this study, OTUD6B is identified as a potent deubiquitinase of β-TrCP that suppress ESCC progression through the OTUD6B-β-TrCP-SNAIL axis. Low OTUD6B expression is associated with a poor prognosis of ESCC patients. Importantly, all-trans retinoic acid (ATRA) is found to promote OTUD6B translation and thus suppress ESCC tumor growth and enhance the response of ESCC tumors to anti-PD-1 immunotherapies. These findings demonstrate that OTUD6B is a crucial deubiquitinase of β-TrCP in ESCC and suggest combination of ATRA and anti-PD-1 immune checkpoint inhibitor may benefit a cohort of ESCC patients.
Collapse
Affiliation(s)
- Lei Li
- State Key Laboratory of Molecular OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijing100021P. R. China
- Department of Radiation OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeShenzhen518116P. R. China
| | - Rui Zhu
- State Key Laboratory of Molecular OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijing100021P. R. China
| | - Honghong Zhou
- State Key Laboratory of Molecular OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijing100021P. R. China
| | - Chun‐Ping Cui
- State Key Laboratory of ProteomicsNational Center for Protein Sciences (Beijing)Beijing Institute of LifeomicsBeijing100850P. R. China
| | - Xiao Yu
- State Key Laboratory of Molecular OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijing100021P. R. China
| | - Yuhao Liu
- State Key Laboratory of Molecular OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijing100021P. R. China
- Department of Radiation OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeShenzhen518116P. R. China
| | - Yin Yin
- State Key Laboratory of Molecular OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijing100021P. R. China
| | - Yang Li
- State Key Laboratory of Molecular OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijing100021P. R. China
| | - Riyue Feng
- State Key Laboratory of Molecular OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijing100021P. R. China
| | - Jonathan P. Katz
- Gastroenterology DivisionDepartment of MedicineUniversity of PennsylvaniaPhiladelphiaPA19104USA
| | - Yahui Zhao
- State Key Laboratory of Molecular OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijing100021P. R. China
| | - Yun Zhang
- State Key Laboratory of Molecular OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijing100021P. R. China
| | - Lingqiang Zhang
- State Key Laboratory of ProteomicsNational Center for Protein Sciences (Beijing)Beijing Institute of LifeomicsBeijing100850P. R. China
| | - Zhihua Liu
- State Key Laboratory of Molecular OncologyNational Cancer Center/National Clinical Research Center for Cancer/Cancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijing100021P. R. China
| |
Collapse
|
|
61
|
Kabir MF, Jackson JL, Fuller AD, Gathuka L, Karami AL, Conde DG, Klochkova A, Mu A, Cai KQ, Klein-Szanto AJ, Muir AB, Whelan KA. Diclofenac exhibits cytotoxic activity associated with metabolic alterations and p53 induction in ESCC cell lines and decreases ESCC tumor burden in vivo. Carcinogenesis 2023; 44:182-195. [PMID: 37014121 PMCID: PMC10215983 DOI: 10.1093/carcin/bgad019] [Citation(s) in RCA: 1] [Impact Index Per Article: 0.5] [Reference Citation Analysis] [Abstract] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/01/2022] [Revised: 03/13/2023] [Accepted: 04/03/2023] [Indexed: 04/05/2023] Open
Abstract
Esophageal squamous cell carcinoma (ESCC) is one of the most aggressive forms of human malignancy, often displaying limited therapeutic response. Here, we examine the non-steroidal anti-inflammatory drug diclofenac (DCF) as a novel therapeutic agent in ESCC using complementary in vitro and in vivo models. DCF selectively reduced viability of human ESCC cell lines TE11, KYSE150, and KYSE410 as compared with normal primary or immortalized esophageal keratinocytes. Apoptosis and altered cell cycle profiles were documented in DCF-treated TE11 and KYSE 150. In DCF-treated TE11, RNA-Sequencing identified differentially expressed genes and Ingenuity Pathway Analysis predicted alterations in pathways associated with cellular metabolism and p53 signaling. Downregulation of proteins associated with glycolysis was documented in DCF-treated TE11 and KYSE150. In response to DCF, TE11 cells further displayed reduced levels of ATP, pyruvate, and lactate. Evidence of mitochondrial depolarization and superoxide production was induced by DCF in TE11 and KYSE150. In DCF-treated TE11, the superoxide scavenger MitoTempo improved viability, supporting a role for mitochondrial reactive oxygen species in DCF-mediated toxicity. DCF treatment resulted in increased expression of p53 in TE11 and KYSE150. p53 was further identified as a mediator of DCF-mediated toxicity in TE11 as genetic depletion of p53 partially limited apoptosis in response to DCF. Consistent with the anticancer activity of DCF in vitro, the drug significantly decreased tumor burdene in syngeneic ESCC xenograft tumors and 4-nitroquinoline 1-oxide-mediated ESCC lesions in vivo. These preclinical findings identify DCF as an experimental therapeutic that should be explored further in ESCC.
Collapse
Affiliation(s)
- Mohammad Faujul Kabir
- Fels Cancer Institute for Personalized Medicine, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA
| | - Jazmyne L Jackson
- Fels Cancer Institute for Personalized Medicine, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA
| | - Annie D Fuller
- Fels Cancer Institute for Personalized Medicine, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA
| | - Leonny Gathuka
- Fels Cancer Institute for Personalized Medicine, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA
| | - Adam L Karami
- Fels Cancer Institute for Personalized Medicine, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA
| | - Don-Gerard Conde
- Fels Cancer Institute for Personalized Medicine, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA
| | - Alena Klochkova
- Fels Cancer Institute for Personalized Medicine, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA
| | - Anbin Mu
- Fels Cancer Institute for Personalized Medicine, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA
| | - Kathy Q Cai
- Histopathology Facility, Fox Chase Cancer Center, Philadelphia, PA, USA
| | | | - Amanda B Muir
- Department of Pediatrics, Division of Gastroenterology, Hepatology, and Nutrition, Children’s Hospital of Philadelphia, Philadelphia, PA, USA
| | - Kelly A Whelan
- Fels Cancer Institute for Personalized Medicine, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA
- Department of Cancer & Cellular Biology, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA
| |
Collapse
|
|
62
|
Zhu H, Yip HC, Cheung MK, Chan HC, Ng C, Lau EHL, Yeung ZWC, Wong EWY, Leung L, Qu X, Wang D, Cai L, Chan PKS, Chan JYK, Chen Z. Convergent dysbiosis of upper aerodigestive microbiota between patients with esophageal and oral cavity squamous cell carcinoma. Int J Cancer 2023; 152:1903-1915. [PMID: 36752573 DOI: 10.1002/ijc.34460] [Citation(s) in RCA: 9] [Impact Index Per Article: 4.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/22/2022] [Revised: 01/16/2023] [Accepted: 01/18/2023] [Indexed: 02/09/2023]
Abstract
The bidirectional association between primary esophageal squamous cell carcinoma (ESCC) and oral cavity squamous cell carcinoma (OSCC) suggests common risk factors and oncogenic molecular processes but it is unclear whether these two cancers display similar patterns of dysbiosis in their upper aerodigestive microbiota (UADM). We conducted a case-control study to characterize the microbial communities in esophageal lavage samples from 49 ESCC patients and oral rinse samples from 91 OSCC patients using 16S rRNA V3-V4 amplicon sequencing. Compared with their respective non-SCC controls from the same anatomical sites, 32 and 45 discriminative bacterial genera were detected in ESCC and OSCC patients, respectively. Interestingly, 20 of them were commonly enriched or depleted in both types of cancer, suggesting a convergent niche adaptation of upper aerodigestive SCC-associated bacteria that may play important roles in the pathogenesis of malignancies. Notably, Fusobacterium, Selenomonas, Peptoanaerobacter and Peptostreptococcus were enriched in both ESCC and OSCC, whereas Streptococcus and Granulicatelia were commonly depleted. We further identified Fusobacterium nucleatum as the most abundant species enriched in the upper aerodigestive SCC microenvironment, and the higher relative abundances of Selenomonas danae and Treponema maroon were positively correlated with smoking. In addition, predicted functional analysis revealed several depleted (eg, lipoic acid and pyruvate metabolism) and enriched (eg, RNA polymerase and nucleotide excision repair) pathways common to both cancers. Our findings reveal a convergent dysbiosis in the UADM between patients with ESCC and OSCC, suggesting a shared niche adaptation of host-microbiota interactions in the pathogenesis of upper aerodigestive tract malignancies.
Collapse
Affiliation(s)
- Hengyan Zhu
- Department of Microbiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Hon Chi Yip
- Department of Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Man Kit Cheung
- Department of Microbiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Hiu Ching Chan
- Department of Microbiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Cherrie Ng
- Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Eric H L Lau
- Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Zenon W C Yeung
- Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Eddy W Y Wong
- Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Leanne Leung
- Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Xinyu Qu
- Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Daijuanru Wang
- Department of Microbiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Liuyang Cai
- Department of Microbiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Paul K S Chan
- Department of Microbiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Jason Y K Chan
- Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| | - Zigui Chen
- Department of Microbiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
| |
Collapse
|
|
63
|
Wang Q, Qin Y, Li B. CD8 + T cell exhaustion and cancer immunotherapy. Cancer Lett 2023; 559:216043. [PMID: 36584935 DOI: 10.1016/j.canlet.2022.216043] [Citation(s) in RCA: 71] [Impact Index Per Article: 35.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/13/2022] [Revised: 12/11/2022] [Accepted: 12/21/2022] [Indexed: 12/28/2022]
Abstract
Immunotherapy plays an increasingly important role in the treatment of most malignant tumors, and CD8+ T cells are the most important antitumor effector cells in the process of immunotherapy, and their number and functional status largely determine the antitumor effect. However, under continuous antigen exposure and the stimulation of inflammatory factors, CD8+ T cells gradually show a weakened proliferation and effector function, accompanied by the expression of a variety of inhibitory receptors. This state is known as CD8+ T cell "exhaustion" and often leads to the loss of control and progression of tumors. Recent studies provided us a better understanding of the mechanisms of T cell exhaustion, this review provides an overview of the activation, exhaustion mechanisms and exhaustion characteristics of CD8+ T cells. Although immunotherapy can reverse the exhaustion of CD8+ T cells and significantly improve the antitumor effects, single immunotherapy often has limitations, and it is difficult to achieve satisfactory antitumor effects, therefore, this review also summarizes up-to-date information related to cancer immunotherapy, and these emerging insights provide promising clues to the future management of malignant tumors.
Collapse
Affiliation(s)
- Qingda Wang
- Department of Liver Surgery, West China Hospital, Sichuan University Medical School, Chengdu, China
| | - Yang Qin
- Department of Biochemistry and Molecular Biology, West China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, Chengdu, China.
| | - Bo Li
- Department of Liver Surgery, West China Hospital, Sichuan University Medical School, Chengdu, China.
| |
Collapse
|
|
64
|
Zhu Q, Xu H, Huang L, Luo J, Li H, Yang R, Liu X, Liu F. Identification and detection of plasma extracellular vesicles-derived biomarkers for esophageal squamous cell carcinoma diagnosis. Biosens Bioelectron 2023; 225:115088. [PMID: 36739741 DOI: 10.1016/j.bios.2023.115088] [Citation(s) in RCA: 18] [Impact Index Per Article: 9.0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/20/2022] [Revised: 01/13/2023] [Accepted: 01/18/2023] [Indexed: 01/22/2023]
Abstract
Esophageal cancer is a malignant tumor with two-thirds of patients having a local recurrence or distant metastasis. To date, diagnostic biomarkers with high sensitivity and specificity are lacking. Extracellular vesicles (EVs) have shown their potential values as disease biomarkers as they carry specific proteins and RNAs derived from cancer cells. In this study, we investigate ESCC precision diagnostics from the insights of circulating EVs, and integrate the ultrafast EV isolation approach (EXODUS) and ELISA for fast detection and screening of ESCC patients. First, we isolate and characterize the high-purity plasma EVs with EXODUS and identify 401 proteins and 372 proteins from ESCC patient and healthy individuals, respectively. Further looking into the differentially expressed proteins (DEPs) of ESCC patients and enriched KEGG pathways, we discover EV-CD14 as a potential diagnostic biomarker for ESCC, which has been further validated as a significantly differentially expressed protein by Western Blot and immunogold labelling TEM. For fast screening and detection of ESCC towards clinical applications, we apply ELISA method to diagnose ESCC from 60 clinical samples based on circulating EV-CD14, which shows a high AUC value up to 96.0% for detection of ESCC in a test set (30 samples), and displays a high accuracy rate up to 90% for prediction of ESCC in a screening test (30 samples). Our results suggest that the circulating EV-CD14 may highly be related to the initiation and progression of ESCC, providing a novel method for the diagnosis and prognosis of ESCC towards clinical translations.
Collapse
Affiliation(s)
- Qingfu Zhu
- National Engineering Research Center of Ophthalmology and Optometry, Eye Hospital, Wenzhou Medical University, Wenzhou, 325027, China
| | - Hao Xu
- National Engineering Research Center of Ophthalmology and Optometry, Eye Hospital, Wenzhou Medical University, Wenzhou, 325027, China
| | - Liu Huang
- Department of Oncology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, China
| | - Jiaxin Luo
- National Engineering Research Center of Ophthalmology and Optometry, Eye Hospital, Wenzhou Medical University, Wenzhou, 325027, China
| | - Hengrui Li
- National Engineering Research Center of Ophthalmology and Optometry, Eye Hospital, Wenzhou Medical University, Wenzhou, 325027, China
| | - Rui Yang
- National Engineering Research Center of Ophthalmology and Optometry, Eye Hospital, Wenzhou Medical University, Wenzhou, 325027, China
| | - Xiaoling Liu
- Center for Biological Science and Technology, Advanced Institute of Natural Sciences, Beijing Normal University at Zhuhai, Zhuhai, China
| | - Fei Liu
- National Engineering Research Center of Ophthalmology and Optometry, Eye Hospital, Wenzhou Medical University, Wenzhou, 325027, China.
| |
Collapse
|
|
65
|
Oguma J, Ishiyama K, Kurita D, Kanematsu K, Kubo K, Utsunomiya D, Yamamoto S, Honma Y, Kato K, Daiko H. Significance of lymphovascular invasion in esophageal squamous cell carcinoma undergoing neoadjuvant chemotherapy followed by esophagectomy. Esophagus 2023; 20:215-224. [PMID: 36565340 DOI: 10.1007/s10388-022-00973-y] [Citation(s) in RCA: 2] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 05/06/2022] [Accepted: 11/28/2022] [Indexed: 12/25/2022]
Abstract
BACKGROUND Lymphovascular invasion (LVI) was previously reported to be an independent factor associated with survival in locally advanced esophageal squamous cell carcinoma (LAESCC) patients receiving neoadjuvant chemotherapy (NAC); however, the detailed clinicopathological significance of LVI remains unclear. This study evaluated the prognostic impact of LVI in patients with LAESCC after NAC with cisplatin and 5-fluorouracil (CF) or docetaxel, cisplatin and 5-fluorouracil (DCF) followed by surgery and in LAESCC patients with recurrence after NAC and surgery. METHODS 438 patients with thoracic LAESCC who had undergone NAC followed by an esophagectomy with three-field lymphadenectomy were assessed using a propensity score matched analysis, and their long-term outcomes were retrospectively reviewed. RESULTS In matched cohort, a multivariate analysis of relapse-free survival (RFS) in the NAC-CF group suggested that ypN (≥ 1, HR = 3.715, p = 0.004) and LVI (positive, HR = 3.366, p = 0.012) were independent factors associated with RFS; in the NAC-DCF group, ypN (≥ 1, HR = 4.829, p < 0.001) was the only independent factor associated with RFS. Comparisons of overall survival (OS) between the ypN + /LVI + group and other groups among patients with recurrence in each NAC regimen showed significant differences in both of NAC groups (p < 0.001, respectively). The ypN + /LVI + group had a significantly poor OS in both an oligometastatic recurrence (OMR) group (p < 0.001) and a non-OMR group (p < 0.001). CONCLUSIONS The present study suggested that the independent factor associated with prognosis of patients with LAESCC after NAC and surgery may differ according to the NAC regimen, and the presence of both ypN and LVI was a prognostic factor for patients with recurrence, including those with OMR. These results might be helpful when deciding on an additional treatment strategy for LAESCC patients.
Collapse
Affiliation(s)
- Junya Oguma
- Esophageal Surgery Division, National Cancer Center Hospital, 5-1-1 Tsukiji Chuo-ku, Tokyo, 104-0045, Japan
| | - Koshiro Ishiyama
- Esophageal Surgery Division, National Cancer Center Hospital, 5-1-1 Tsukiji Chuo-ku, Tokyo, 104-0045, Japan
| | - Daisuke Kurita
- Esophageal Surgery Division, National Cancer Center Hospital, 5-1-1 Tsukiji Chuo-ku, Tokyo, 104-0045, Japan
| | - Kyohei Kanematsu
- Esophageal Surgery Division, National Cancer Center Hospital, 5-1-1 Tsukiji Chuo-ku, Tokyo, 104-0045, Japan
| | - Kentaro Kubo
- Esophageal Surgery Division, National Cancer Center Hospital, 5-1-1 Tsukiji Chuo-ku, Tokyo, 104-0045, Japan
| | - Daichi Utsunomiya
- Esophageal Surgery Division, National Cancer Center Hospital, 5-1-1 Tsukiji Chuo-ku, Tokyo, 104-0045, Japan
| | - Shun Yamamoto
- Department Head and Neck, Esophageal Medical Oncology, National Cancer Center Hospital, Tokyo, Japan
| | - Yoshitaka Honma
- Department Head and Neck, Esophageal Medical Oncology, National Cancer Center Hospital, Tokyo, Japan
| | - Ken Kato
- Department Head and Neck, Esophageal Medical Oncology, National Cancer Center Hospital, Tokyo, Japan
| | - Hiroyuki Daiko
- Esophageal Surgery Division, National Cancer Center Hospital, 5-1-1 Tsukiji Chuo-ku, Tokyo, 104-0045, Japan.
| |
Collapse
|
|
66
|
Liu Y, Wu M, Xu S, Niu X, Liu W, Miao C, Lin A, Xu Y, Yu L. PSMD2 contributes to the progression of esophageal squamous cell carcinoma by repressing autophagy. Cell Biosci 2023; 13:67. [PMID: 36998052 DOI: 10.1186/s13578-023-01016-4] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/29/2022] [Accepted: 03/16/2023] [Indexed: 04/01/2023] Open
Abstract
BACKGROUND The ubiquitin-proteasome and autophagy-lysosomal systems collaborate in regulating the levels of intracellular proteins. Dysregulation of protein homeostasis is a central feature of malignancy. The gene encoding 26S proteasome non-ATPase regulatory subunit 2 (PSMD2) of the ubiquitin-proteasome system is an oncogene in various types of cancer. However, the detailed role of PSMD2 in autophagy and its relationship to tumorigenesis in esophageal squamous cell carcinoma (ESCC) remain unknown. In the present study, we have investigated the tumor-promoting roles of PSMD2 in the context of autophagy in ESCC. METHODS Molecular approaches including DAPgreen staining, 5-Ethynyl-2'-deoxyuridine (EdU), cell counting kit 8 (CCK8), colony formation, transwell assays, and cell transfection, xenograft model, immunoblotting and Immunohistochemical analysis were used to investigate the roles of PSMD2 in ESCC cells. Data-independent acquisition (DIA) quantification proteomics analysis and rescue experiments were used to study the roles of PSMD2 in ESCC cells. RESULTS We demonstrate that the overexpression of PSMD2 promotes ESCC cell growth by inhibiting autophagy and is correlated with tumor progression and poor prognosis of ESCC patients. DIA quantification proteomics analysis shows a significant positive correlation between argininosuccinate synthase 1 (ASS1) and PSMD2 levels in ESCC tumors. Further studies indicate that PSMD2 activates the mTOR pathway by upregulating ASS1 to inhibit autophagy. CONCLUSIONS PSMD2 plays an important role in repressing autophagy in ESCC, and represents a promising biomarker to predict prognosis and a therapeutic target of ESCC patients.
Collapse
Affiliation(s)
- Yachen Liu
- Department of Thoracic Surgery, Fudan University Shanghai Cancer Center, Shanghai, China
- Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- Department of Etiology and Carcinogenesis, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People's Republic of China
| | - Meng Wu
- Department of Cardiology, Cardiovascular Key Lab of Zhejiang Province, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310009, China
- Department of Medical Oncology, Key Laboratory of Cancer Prevention and Intervention, Ministry of Education, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310009, China
| | - Shuxiang Xu
- Department of Cardiology, Cardiovascular Key Lab of Zhejiang Province, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310009, China
| | - Xiangjie Niu
- Department of Etiology and Carcinogenesis, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People's Republic of China
| | - Weiling Liu
- Department of Etiology and Carcinogenesis, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People's Republic of China
| | - Chuanwang Miao
- Department of Etiology and Carcinogenesis, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People's Republic of China
| | - Ai Lin
- Department of Etiology and Carcinogenesis, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People's Republic of China
| | - Yang Xu
- Department of Cardiology, Cardiovascular Key Lab of Zhejiang Province, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310009, China.
| | - Lili Yu
- Department of Medical Oncology, Key Laboratory of Cancer Prevention and Intervention, Ministry of Education, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310009, China.
| |
Collapse
|
|
67
|
Fan Z, Liu Y, Liu X, Nian W, Huang X, Yang Q, Hou S, Chen F. Phosphorylation of AKT by lysyl oxidase-like 2 activates the PI3K/AKT signaling pathway to promote proliferation, invasion and metastasis in esophageal squamous carcinoma. Clin Transl Oncol 2023:10.1007/s12094-023-03133-5. [PMID: 36995521 DOI: 10.1007/s12094-023-03133-5] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Grants] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/30/2022] [Accepted: 02/19/2023] [Indexed: 03/31/2023]
Abstract
OBJECTIVE Esophageal squamous cell carcinoma (ESCC) is a common and aggressive malignancy of the gastrointestinal tract for which therapeutic options are scarce. This study screens for LOXL2, a key gene in ESCC, and explains the molecular mechanism by which it promotes the progression of ESCC. METHODS Immunohistochemical staining was performed to detect the expression level of LOXL2 in ESCC tissues and paraneoplastic tissues. CCK-8 and Transwell assays were performed to assess the effects of LOXL2 knockdown and overexpression on the proliferation, apoptosis, migration and invasion ability of ESCC cells. High-throughput sequencing analysis screens for molecular mechanisms of action by which LOXL2 promotes ESCC progression. Western blotting and qRT-PCR were used to determine the expression levels of relevant markers. RESULTS LOXL2 is positively expressed in ESCC and highly correlated with poor prognosis. Silencing LOXL2 significantly inhibited the proliferation, migration and invasive ability of ESCC cells, whereas overexpression showed the opposite phenotype. High-throughput sequencing suggested that LOXL2-associated differentially expressed genes were highly enriched in the PI3K/AKT signaling pathway. In vitro cellular assays confirmed that silencing LOXL2 significantly reduced PI3K, p-AKTThr308 and p-AKTSer473 gene and protein expression levels, while overexpression increased all three gene and protein levels, while AKT gene and protein expression levels were not significantly different. CONCLUSION This study found that LOXL2 may regulate the PI3K/AKT signaling pathway and exert protumor effects on ESCC cells through phosphorylation of AKT. LOXL2 may be a key clinical warning biomarker or therapeutic target for ESCC.
Collapse
Affiliation(s)
- Zhiqin Fan
- Department of Daily Surgery, Affiliated Tumor Hospital, Xinjiang Medical University, Urumqi, China
- State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia, Xinjiang Medical University, Urumqi, China
| | - Yingmin Liu
- Department of Daily Surgery, Affiliated Tumor Hospital, Xinjiang Medical University, Urumqi, China
| | - Xinya Liu
- Department of Cardiac Oncology Disease, Affiliated Tumor Hospital, Xinjiang Medical University, Urumqi, China
| | - Wei Nian
- Department of Daily Surgery, Affiliated Tumor Hospital, Xinjiang Medical University, Urumqi, China
| | - Xiaotong Huang
- Department of Daily Surgery, Affiliated Tumor Hospital, Xinjiang Medical University, Urumqi, China
| | - Qianqian Yang
- Department of Daily Surgery, Affiliated Tumor Hospital, Xinjiang Medical University, Urumqi, China
| | - Songyu Hou
- Department of Daily Surgery, Affiliated Tumor Hospital, Xinjiang Medical University, Urumqi, China
| | - Fei Chen
- Department of Daily Surgery, Affiliated Tumor Hospital, Xinjiang Medical University, Urumqi, China.
| |
Collapse
|
|
68
|
Surgical outcomes of reconstruction using the gastric tube and free jejunum for cervical esophageal cancer: analysis using the National Clinical Database of Japan. Esophagus 2023:10.1007/s10388-023-00997-y. [PMID: 36899133 DOI: 10.1007/s10388-023-00997-y] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 09/23/2022] [Accepted: 03/03/2023] [Indexed: 03/12/2023]
Abstract
BACKGROUND Cervical esophageal cancer accounts for a small proportion of all esophageal cancers. Therefore, studies examining this cancer include a small patient cohort. Most patients with cervical esophageal cancer undergo reconstruction using a gastric tube or free jejunum after esophagectomy. We examined the current status of postoperative morbidity and mortality of cervical esophageal cancer based on big data. METHODS Based on the Japan National Clinical Database, 807 surgically treated patients with cervical esophageal cancer were enrolled between January 1, 2016, and December 31, 2019. Surgical outcomes were retrospectively reviewed for each reconstructed organ using gastric tubes and free jejunum. RESULTS The incidence of postoperative complications related to reconstructed organs was higher in the gastric tube reconstruction (17.9%) than in the free jejunum (6.7%) for anastomotic leakage (p < 0.01), but not significantly different for reconstructed organ necrosis (0.4% and 0.3%, respectively). The incidence rates of overall morbidity, pneumonia, 30-day reoperation, tracheal necrosis, and 30-day mortality using these reconstruction methods were 64.7% and 59.7%, 16.7% and 11.1%, 9.3% and 11.4%, 2.2% and 1.6%, and 1.2% and 0.0%, respectively. Only pneumonia was more common in the gastric tube reconstruction group (p = 0.03), but was not significantly different for any other complication. CONCLUSIONS The incidence of overall morbidities and reoperation, especially anastomotic leakage after gastric tube reconstruction, suggested a necessity for further improvement. However, the incidence of fatal complications, such as tracheal necrosis or reconstructed organ necrosis, was low for both reconstruction methods, and the mortality rate was acceptable as a means of radical treatment.
Collapse
|
|
69
|
Xu X, Jiang F, Guo Y, Chen H, Qian J, Wu L, Xie D, Chen G. Clinical-Pathological Characteristics of Adenosquamous Esophageal Carcinoma: A Propensity-Score-Matching Study. J Pers Med 2023; 13:468. [PMID: 36983650 PMCID: PMC10057829 DOI: 10.3390/jpm13030468] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 02/06/2023] [Revised: 02/24/2023] [Accepted: 03/01/2023] [Indexed: 03/08/2023] Open
Abstract
There are few studies on esophageal adenosquamous carcinoma (ADSC). Our study intended to investigate the clinical and survival features of ADSC. We included esophageal cancer (EC) data from the Surveillance, Epidemiology, and End Results program database to explore clinical and survival traits. Propensity score matching (PSM), the multivariate Cox regression model, and survival curves were used in this study. A total of 137 patients with ADSC were included in our analysis. The proportion of ADSC within the EC cohort declined from 2004 to 2018. Besides, results indicated no significant difference in survival between ADSC and SCC groups (PSM-adjusted HR = 1.249, P = 0.127). However, the survival rate of the ADSC group was significantly worse than that of the ADC group (PSM-adjusted HR = 1.497, P = 0.007). For the ADSC group, combined treatment with surgery had a higher survival rate than other treatment methods (all P < 0.001). Surgical resection, radiotherapy, and chemotherapy were independent protective prognostic factors (all P < 0.05). The proportion of ADSC has been declining from 2004 to 2018. The prognosis of ADSC is not significantly different from that of SCC but is worse than that of ADC. Surgery, radiotherapy, and chemotherapy could improve the prognosis of patients. Comprehensive treatment with surgery as the main treatment is more beneficial for some patients.
Collapse
Affiliation(s)
- Xinxin Xu
- Department of Gastroenterology, The Affiliated Clinical College of Xuzhou Medical University, Xuzhou 221002, China
| | - Feng Jiang
- Department of Oncology, Zhongda Hospital, Southeast University, Nanjing 210009, China
| | - Yihan Guo
- Department of Scientific Research, Shaanxi Academy of Social Sciences, Xi’an 710061, China
| | - Hu Chen
- Department of Gastroenterology, The Affiliated Clinical College of Xuzhou Medical University, Xuzhou 221002, China
| | - Jiayi Qian
- Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University, Shanghai 200092, China
| | - Leilei Wu
- Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University, Shanghai 200092, China
| | - Dong Xie
- Department of Thoracic Surgery, Shanghai Pulmonary Hospital, Tongji University, Shanghai 200092, China
| | - Guangxia Chen
- Department of Gastroenterology, The Affiliated Xuzhou Municipal Hospital of Xuzhou Medical University, Xuzhou 221002, China
| |
Collapse
|
|
70
|
He XY, Wang XQ, Xiao QL, Liu D, Xu QR, Liu S. Long non-coding RNA NCK1-AS1 functions as a ceRNA to regulate cell viability and invasion in esophageal squamous cell carcinoma via microRNA-133b/ENPEP axis. Cell Cycle 2023; 22:596-609. [PMID: 36412985 PMCID: PMC9928473 DOI: 10.1080/15384101.2022.2138416] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 02/07/2021] [Revised: 01/04/2022] [Accepted: 10/17/2022] [Indexed: 11/23/2022] Open
Abstract
This study is designed to explore the role of long non-coding RNAs (lncRNAs) NCK1-AS1 in proliferative and invasive activities of esophageal squamous cell carcinoma (ESCC) cells by binding to microRNA-133b (miR-133b) to regulate ENPEP. Differentially expressed lncRNAs, miRs, genes and their targeting relationships were screened on ESCC-related gene expression datasets GSE17351 and GSE6188. The targeting relationships among NCK1-AS1, miR-133b, and ENPEP were verified using functional assays. Loss- and gain- of function assays were carried out to examine the roles of NCK1-AS1, miR-133b, and ENPEP in ESCC cell proliferative, invasive, migrative and apoptotic abilities as well as tumorigenesis in vivo. Elevated NCK1-AS1 and ENPEP but reduced miR-133b expression were found in ESCC. NCK1-AS1 knockdown or miR-133b overexpression inhibited the malignant properties of ESCC cells as well as tumorigenesis in vivo. NCK1-AS1 regulated the ENPEP expression by competitively binding to miR-133b. ENPEP overexpression reversed inhibition of NCK1-AS1 knockdown on the function of ESCC cells. This study provides evidence that silencing NCK1-AS1 inhibits expression of ENPEP by sponging miR-133b, thereby suppressing ESCC.
Collapse
Affiliation(s)
- Xiang-Yuan He
- Department of Thoracic Surgery, The First Affiliated Hospital of Nanchang University, Nanchang, P. R. China
| | - Xiu-Qi Wang
- Department of Thoracic Surgery, The First Affiliated Hospital of Nanchang University, Nanchang, P. R. China
| | - Qi-Lu Xiao
- Department of Thoracic Surgery, The First Affiliated Hospital of Nanchang University, Nanchang, P. R. China
| | - Duan Liu
- Department of Thoracic Surgery, The First Affiliated Hospital of Nanchang University, Nanchang, P. R. China
| | - Qi-Rong Xu
- Department of Thoracic Surgery, The First Affiliated Hospital of Nanchang University, Nanchang, P. R. China
| | - Sheng Liu
- Department of Thoracic Surgery, The First Affiliated Hospital of Nanchang University, Nanchang, P. R. China
| |
Collapse
|
|
71
|
Wang X, Zhang W, Wu W, Wu S, Young A, Yan Z. Is Candida albicans a contributor to cancer? A critical review based on the current evidence. Microbiol Res 2023; 272:127370. [PMID: 37028206 DOI: 10.1016/j.micres.2023.127370] [Citation(s) in RCA: 20] [Impact Index Per Article: 10.0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/26/2022] [Revised: 03/23/2023] [Accepted: 03/26/2023] [Indexed: 04/01/2023]
Abstract
The association between Candida albicans (C. albicans) and cancer has been noticed for decades. Whether C. albicans infection is a complication of cancer status or as a contributor to cancer development remains to be discussed. This review systematically summarized the up-to-date knowledge about associations between C. albicans and various types of cancer, and discussed the role of C. albicans in cancer development. Most of the current clinical and animal evidence support the relationship between C. albicans and oral cancer development. However, there is insufficient evidence to demonstrate the role of C. albicans in other types of cancer. Moreover, this review explored the underlying mechanisms for C. albicans promoting cancer. It was hypothesized that C. albicans may promote cancer progression by producing carcinogenic metabolites, inducing chronic inflammation, remodeling immune microenvironment, activating pro-cancer signals, and synergizing with bacteria.
Collapse
|
|
72
|
Wang H, Jiang Z, Wang Q, Wu T, Guo F, Xu Z, Yang W, Yang S, Feng S, Wang X, Chen S, Cheng C, Chen W. Pathological response and prognostic factors of neoadjuvant PD-1 blockade combined with chemotherapy in resectable esophageal squamous cell carcinoma. Eur J Cancer 2023; 186:196-210. [PMID: 37045666 DOI: 10.1016/j.ejca.2023.03.008] [Citation(s) in RCA: 10] [Impact Index Per Article: 5.0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/10/2023] [Revised: 03/09/2023] [Accepted: 03/10/2023] [Indexed: 03/18/2023]
Abstract
PURPOSE We aimed to investigate the pathological changes, clinicopathological correlation and prognostic factors of neoadjuvant programmed cell death 1 (PD-1) blockade camrelizumab combined with carboplatin and nab-paclitaxel (CCNP) which we have proved its effectiveness in previous research for resectable esophageal squamous cell carcinoma (ESCC). METHODS 108 patients of resectable ESCC, with a mean follow-up of 13 m (ranging 1-30 m), treated with neoadjuvant CCNP from March 2020 to October 2022 in the First Affiliated Hospital of Sun Yat-sen University were enrolled. RESULTS One year overall survival (OS) and disease-free survival (DFS) were 96.4% and 84.7% respectively. Pathological complete response or major pathological response (pCR/MPR) of the primary tumour (T-pCR/T-MPR) and the metastatic lymph node (N-pCR/N-MPR) were 58.3% and 47.5%. Pathological response of both primary tumours (PT) and lymph nodes (LN) metastasis correlated with DFS. LN pathological response was consistent with PT in 70.0% and inconsistent in 30.0% metastatic cases. Higher ratio of CD8+ to FoxP3+ tumour-infiltrating lymphocytes (TILs), earlier ypT stage and PT invasion not beyond circular muscle correlated with better pathological response. Four types of regression patterns of PT and two types of metastatic LN regression were found. A total of 18 (16.7%) out of 108 developed recurrence with a mean time of 6.9 ± 5.3 months. PT pathological response plus ypN and PT invasion beyond circular muscle or not were independent prognostic factors of DFS. CONCLUSIONS This study suggested that camrelizumab plus chemotherapy had a high rate of T-pCR/T-MPR for resectable ESCC. T-pCR/T-MPR plus ypN0 and tumour invasion not beyond circular muscle predicted better DFS.
Collapse
|
|
73
|
Liao G, Tang J, Bai J. Early development of esophageal squamous cell cancer: Stem cells, cellular origins and early clone evolution. Cancer Lett 2023; 555:216047. [PMID: 36587837 DOI: 10.1016/j.canlet.2022.216047] [Citation(s) in RCA: 6] [Impact Index Per Article: 3.0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/04/2022] [Revised: 12/08/2022] [Accepted: 12/24/2022] [Indexed: 12/31/2022]
Abstract
Esophageal squamous cell carcinoma (ESCC), a highly malignant cancer with poor prognosis, is an example of the classical view of cancer development based on stem cell origin and multistep progression. In the past five years, the applications of large-scale sequencing and single-cell sequencing have expanded to human esophageal normal tissues and precancerous lesions, which, coupled with the application of transgenic lineage tracing technology in mouse models, has provided a more comprehensive and detailed understanding of esophageal stem cell heterogeneity and early clonal evolution of ESCC. In this review, we discuss the heterogeneity of esophageal basal-layer stem cells and their potential relationship with cells of ESCC origin. We present evidence that expansion of NOTCH1 mutants may call into play an evolutionarily conserved anti-cancer mechanism and mold the model of early clonal evolution in ESCCs. Finally, we discuss the potential avenues in this context. This review provides a focused understanding of the early development of ESCC, as a background for early tumor detection, intervention, and prevention strategies.
Collapse
Affiliation(s)
- Guobin Liao
- Department of Gastroenterology, Second Affiliated Hospital, Army Medical University, Chongqing, 400037, China; Department of Gastroenterology, The 901 Hospital of Chinese People's Liberation Army Joint Service Support Unit, Hefei, 230000, China.
| | - Jun Tang
- Department of Gastroenterology, The 901 Hospital of Chinese People's Liberation Army Joint Service Support Unit, Hefei, 230000, China.
| | - Jianying Bai
- Department of Gastroenterology, Second Affiliated Hospital, Army Medical University, Chongqing, 400037, China.
| |
Collapse
|
|
74
|
Cost-effectiveness of toripalimab plus chemotherapy for advanced esophageal squamous cell carcinoma. Int J Clin Pharm 2023:10.1007/s11096-023-01540-w. [PMID: 36800145 DOI: 10.1007/s11096-023-01540-w] [Citation(s) in RCA: 8] [Impact Index Per Article: 4.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/08/2022] [Accepted: 01/09/2023] [Indexed: 02/18/2023]
Abstract
BACKGROUND Toripalimab is an immune checkpoint inhibitor (ICI) against programmed death ligand 1 (PD-L1). It has been approved for advanced esophageal squamous cell carcinoma (ESCC) as the first-line treatment due to significantly improved progression-free survival (PFS) and overall survival (OS) in the JUPITER-06 trial. AIM This study aimed to compare the cost-effectiveness between toripalimab plus chemotherapy and placebo plus chemotherapy from the perspective of the Chinese health system. METHOD The study developed a 3-year partitioned survival model to assess costs and outcomes in two treatment groups with or without toripalimab. The critical indicator was the incremental cost-effectiveness ratio (ICER). Scenario and sensitivity analyses were performed to evaluate the robustness of the findings and identify the parameters with the greatest impact on cost-effectiveness. RESULTS In the base case analysis, the incremental effectiveness and cost of toripalimab plus chemotherapy versus placebo plus chemotherapy were 0.26 quality-adjusted life year (QALYs) and $11,254.84, respectively, resulting in an ICER of $43,405.09/QALY, higher than the 2021 willingness-to-pay threshold in China ($37,658.70/QALY). The results were sensitive to the utility of PFS, the incidence of neutropenia in the toripalimab group, and the cost of toripalimab. The toripalimab plus chemotherapy group was cost-effective only if the price of toripalimab decreased by more than 40%. CONCLUSION Adding toripalimab to chemotherapy was not cost-effective in patients with advanced ESCC in China.
Collapse
|
|
75
|
Association of LAMA1 Single-Nucleotide Polymorphisms with Risk of Esophageal Squamous Cell Carcinoma among the Eastern Chinese Population. JOURNAL OF ONCOLOGY 2023; 2023:6922909. [PMID: 36824663 PMCID: PMC9943613 DOI: 10.1155/2023/6922909] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Figures] [Subscribe] [Scholar Register] [Received: 09/04/2022] [Revised: 10/03/2022] [Accepted: 10/10/2022] [Indexed: 02/18/2023]
Abstract
Introduction LAMA1, also known as laminin subunit α1, is a member of the laminin family, which is widely reported to be a key basement membrane molecule that affects various biological activities and is associated with many kinds of diseases. We aimed to investigate the association between LAMA1single-nucleotide polymorphisms and the occurrence and progression of esophageal squamous cell carcinoma in the Chinese population. Method 2,186 participants were collected retrospectively between October 2008 and January 2017, including 1,043 ESCC patients and 1,143 noncancer patients. A 2 mL blood sample was obtained intravenously for the LDR for SNP analysis. The 6 SNP loci of LAMA1 were selected and examined. We analyzed the association of several genetic models of 6 LAMA1 SNP loci, sex, age, smoking and drinking status, and the occurrence of esophageal squamous cell carcinoma. Results In the rs62081531 G > A locus, genotype GA was a protective factor for ESCC compared with GG (OR: 0.830, P=0.046), especially among the younger and nondrinkers. At rs607230 T > C, genotype TC was linked with a lower risk of ESCC compared with TT. (OR: 0.613, P=0.034). Haplotype Frequencies revealed that Ars62081531Grs621993Ars539713Trs566655Ars73938538Crs607230 (OR: 0.803, P=0.028) and Grs62081531Grs621993Ars539713Trs566655Crs73938538Crs607230 (OR: 0.679, P=0.010) were strongly associated with lower susceptibility of ESCC. Conclusion The LAMA1 rs62081531, rs539713, rs566655, and rs607230 polymorphisms were demonstrated to be related to susceptibility to ESCC in the Chinese population. LAMA1 SNPs may have a significant impact on the occurrence of esophageal cancer and may serve as potential diagnostic biomarkers.
Collapse
|
|
76
|
Cheng W, Yang F, Ma Y. lncRNA TPT1-AS1 promotes cell migration and invasion in esophageal squamous-cell carcinomas by regulating the miR-26a/HMGA1 axis. Open Med (Wars) 2023; 18:20220533. [PMID: 36820066 PMCID: PMC9938642 DOI: 10.1515/med-2022-0533] [Citation(s) in RCA: 6] [Impact Index Per Article: 3.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/14/2021] [Revised: 07/01/2022] [Accepted: 07/11/2022] [Indexed: 02/16/2023] Open
Abstract
lncRNA TPT1-AS1 plays an oncogenic role in ovarian and cervical cancers. However, its involvement in the pathological progress of esophageal squamous-cell carcinomas (ESCCs) is unclear. lncRNA TPT1-AS1 was mainly localized in the cytoplasm of ESCC cells and interacted with miR-26a. In ESCC tissues, lncRNA TPT1-AS1 level was obviously increased, while miR-26a level was decreased. Interestingly, lncRNA TPT1-AS1 level was not significantly correlated with miR-26a level but was positively correlated with HMGA1 mRNA, a target of miR-26a. In ESCC cell lines KYSE510 and KYSE-30, lncRNA TPT1-AS1 overexpression enhanced HMGA1 expression, while it had no effect on miR-26a expression. Cell migration and proliferation assays indicated that lncRNA TPT1-AS1 and HMGA1 overexpression promoted ESCC cell migration and invasion, while their effects were alleviated by miR-26a overexpression. The migration and invasion of ESCC cells were suppressed by lncRNA TPT1-AS1 knockdown. In conclusion, lncRNA TPT1-AS1 plays an oncogenic role in ESCC and might function by upregulating HMGA1 via sponging miR-26a.
Collapse
Affiliation(s)
- Wenhua Cheng
- The 3rd Department of Digestion, Shanxi Province Cancer Hospital, Shanxi Hospital Affifiliated to Cancer Hospital, Chinese Academy of Medical Sciences, Cancer Hospital Affifiliated to Shanxi Medical University, Taiyuan City, Shanxi Province, 030013, P. R. China
| | - Fang Yang
- Radiotherapy Head and Neck Department, Shanxi Province Cancer Hospital, Shanxi Hospital Affifiliated to Cancer Hospital, Chinese Academy of Medical Sciences, Cancer Hospital Affifiliated to Shanxi Medical University, Taiyuan City, Shanxi Province, 030013, P. R. China
| | - Yong Ma
- The 2nd Department of Chest Surgery, Shanxi Province Cancer Hospital, Shanxi Hospital Affifiliated to Cancer Hospital, Chinese Academy of Medical Sciences, Cancer Hospital Affifiliated to Shanxi Medical University, No. 3 Workers Xin Jie, Xinghualing District, Taiyuan City, Shanxi Province, 030013, P. R. China
| |
Collapse
|
|
77
|
Hu T, Peng H, Yang F, Zhang F, He J. Circ_0024108 promotes the progression of esophageal cancer cells. Gen Thorac Cardiovasc Surg 2023:10.1007/s11748-023-01909-8. [PMID: 36757626 DOI: 10.1007/s11748-023-01909-8] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/25/2022] [Accepted: 01/18/2023] [Indexed: 02/10/2023]
Abstract
BACKGROUND Esophageal squamous cell carcinoma (ESCC) is a serious malignant cancer. The treatment effect of ESCC is relatively poor and needs further improvement. According to reports, circular RNAs (circRNAs) actively participate in human carcinogenesis. More explorations are needed about the action of circRNAs in ESCC. METHODS Circ_0024108, miR-488-3p, and USP14 was quantified by a qRT-PCR or immunoblotting method. Cell proliferation evaluation was performed by MTT, EdU, and colony formation assays. Evaluation of cell motility and invasiveness was conducted using wound healing assay and transwell assay. The regulatory mechanism of circ_0024108, miR-488-3p, and USP14 was detected by RNA pull-down assay and dual-luciferase reporter assay. RESULTS Circ_0024108 and USP14 were significantly overexpressed in ESCC, while miR-488-3p was underexpressed. Deficiency of circ_0024108 impeded cell growth, motility, and invasiveness. Circ_0024108 regulated the expression of USP14 in ESCC cells via miR-488-3p. Also, circ_0024108 was present at high levels in serum exosomes from ESCC patients with high specificity and sensitivity. CONCLUSIONS Taken together, circ_0024108 participated in the progress of ESCC through the miR-488-3p/USP14 axis. Circ_0024108 was differentially expressed in serum exosomes. Circ_0024108 might be a potential biomarker for the diagnosis of ESCC.
Collapse
Affiliation(s)
- Tongchen Hu
- Department of Thoracic Surgery, The Affiliated Hospital of Southwest Medical University, No. 25 TaiPing St, Jiangyang District, Luzhou, 646000, Sichuan, People's Republic of China
| | - Huali Peng
- Department of Thoracic Surgery, The People's Hospital of Leshan, Leshan, 614000, Sichuan, China
| | - Fan Yang
- Department of Thoracic Surgery, The People's Hospital of Leshan, Leshan, 614000, Sichuan, China
| | - Fan Zhang
- Department of Thoracic Surgery, The People's Hospital of Leshan, Leshan, 614000, Sichuan, China
| | - Jintao He
- Department of Thoracic Surgery, The Affiliated Hospital of Southwest Medical University, No. 25 TaiPing St, Jiangyang District, Luzhou, 646000, Sichuan, People's Republic of China.
| |
Collapse
|
|
78
|
Wang XY, Beeraka NM, Xue NN, Yu HM, Yang Y, Liu MX, Nikolenko VN, Liu JQ, Zhao D. Identification of a three-gene prognostic signature for radioresistant esophageal squamous cell carcinoma. World J Clin Oncol 2023; 14:13-26. [PMID: 36699628 PMCID: PMC9850665 DOI: 10.5306/wjco.v14.i1.13] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 07/25/2022] [Revised: 10/25/2022] [Accepted: 12/06/2022] [Indexed: 01/10/2023] Open
Abstract
BACKGROUND Esophageal squamous cell carcinoma (ESCC) is causing a high mortality rate due to the lack of efficient early prognosis markers and suitable therapeutic regimens. The prognostic role of genes responsible for the acquisition of radioresistance in ESCC has not been fully elucidated.
AIM To establish a prognostic model by studying gene expression patterns pertinent to radioresistance in ESCC patients.
METHODS Datasets were obtained from the Gene Expression Omnibus and The Cancer Genome Atlas databases. The edgeR, a Bioconductor package, was used to analyze mRNA expression between different groups. We screened genes specifically responsible for radioresistance to estimate overall survival. Pearson correlation analysis was performed to confirm whether the expression of those genes correlated with each other. Genes contributing to radioresistance and overall survival were assessed by the multivariate Cox regression model through the calculation of βi and risk score using the following formula: 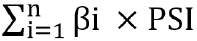 . .
RESULTS We identified three prognostic mRNAs (cathepsin S [CTSS], cluster of differentiation 180 [CD180], and SLP adapter and CSK-interacting membrane protein [SCIMP]) indicative of radioresistance. The expression of the three identified mRNAs was related to each other (r > 0.70 and P < 0.05). As to 1-year and 3-year overall survival prediction, the area under the time-dependent receiver operating characteristic curve of the signature consisting of the three mRNAs was 0.716 and 0.841, respectively. When stratifying patients based on the risk score derived from the signature, the high-risk group exhibited a higher death risk and shorter survival time than the low-risk group (P < 0.0001). Overall survival of the low-risk patients was significantly better than that of the high-risk patients (P = 0.018).
CONCLUSION We have developed a novel three-gene prognostic signature consisting of CTSS, CD180, and SCIMO for ESCC, which may facilitate the prediction of early prognosis of this malignancy.
Collapse
Affiliation(s)
- Xiao-Yan Wang
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Narasimha M Beeraka
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- Department of Pharmaceutical Chemistry, JSS College of Pharmacy, Mysuru 570015, India
| | - Nan-Nan Xue
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Hui-Ming Yu
- Department of Radiation Oncology, Peking University Cancer Hospital & Institute, Beijing 065005, China
| | - Ya Yang
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Mao-Xing Liu
- Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Gastrointestinal Surgery IV, Peking University Cancer Hospital & Institute, Beijing, China
| | - Vladimir N Nikolenko
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- M.V. Lomonosov Moscow State University, Moscow 119991, Russia
| | - Jun-Qi Liu
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Di Zhao
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| |
Collapse
|
|
79
|
Zhang X, Xing M, Ma Y, Zhang Z, Qiu C, Wang X, Zhao Z, Ji Z, Zhang JY. Oridonin Induces Apoptosis in Esophageal Squamous Cell Carcinoma by Inhibiting Cytoskeletal Protein LASP1 and PDLIM1. Molecules 2023; 28:805. [PMID: 36677861 PMCID: PMC9862004 DOI: 10.3390/molecules28020805] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/18/2022] [Revised: 01/07/2023] [Accepted: 01/09/2023] [Indexed: 01/15/2023] Open
Abstract
Esophageal squamous cell carcinoma is a severe malignancy for its high mortality and poor prognosis. Mainstay chemotherapies cause serious side effects for their ways of inducing cell death. Oridonin is the main bioactive constituent from natural plants that has anticancer ability and weak side effects. The proteomics method is efficient to understand the anticancer mechanism. However, proteins identified by proteomics aimed at understanding oridonin's anticancer mechanism is seldom overlapped by different groups. This study used proteomics based on two-dimensional electrophoresis sodium dodecyl sulfate-polyacrylamide gel electrophoresis (2-DE SDS-PAGE) integrated with mass spectrometry and Gene Set Enrichment Analysis (GSEA) to understand the anticancer mechanism of oridonin on esophageal squamous cell carcinoma (ESCC). The results showed that oridonin induced ESCC cell death via apoptosis by decreasing the protein expression of LASP1 and PDLIM1.
Collapse
Affiliation(s)
- Xiaojun Zhang
- Department of Biological Sciences & Border Biomedical Research Center, University of Texas at El Paso, El Paso, TX 79968, USA
- Henan Institute of Medical and Pharmaceutical Sciences, Zhengzhou University, Zhengzhou 450052, China
| | - Mengtao Xing
- Department of Biological Sciences & Border Biomedical Research Center, University of Texas at El Paso, El Paso, TX 79968, USA
| | - Yangcheng Ma
- Department of Biological Sciences & Border Biomedical Research Center, University of Texas at El Paso, El Paso, TX 79968, USA
| | - Zhuangli Zhang
- Henan Institute of Medical and Pharmaceutical Sciences, Zhengzhou University, Zhengzhou 450052, China
| | - Cuipeng Qiu
- Department of Biological Sciences & Border Biomedical Research Center, University of Texas at El Paso, El Paso, TX 79968, USA
| | - Xiao Wang
- Department of Biological Sciences & Border Biomedical Research Center, University of Texas at El Paso, El Paso, TX 79968, USA
| | - Zhihong Zhao
- Henan Institute of Medical and Pharmaceutical Sciences, Zhengzhou University, Zhengzhou 450052, China
| | - Zhenyu Ji
- Henan Institute of Medical and Pharmaceutical Sciences, Zhengzhou University, Zhengzhou 450052, China
| | - Jian-Ying Zhang
- Department of Biological Sciences & Border Biomedical Research Center, University of Texas at El Paso, El Paso, TX 79968, USA
| |
Collapse
|
|
80
|
Kitamura Y, Koma YI, Tanigawa K, Tsukamoto S, Azumi Y, Miyako S, Urakami S, Kodama T, Nishio M, Shigeoka M, Kakeji Y, Yokozaki H. Roles of IL-7R Induced by Interactions between Cancer Cells and Macrophages in the Progression of Esophageal Squamous Cell Carcinoma. Cancers (Basel) 2023; 15:394. [PMID: 36672342 PMCID: PMC9856499 DOI: 10.3390/cancers15020394] [Citation(s) in RCA: 5] [Impact Index Per Article: 2.5] [Reference Citation Analysis] [Abstract] [Key Words] [Grants] [Track Full Text] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/26/2022] [Revised: 12/30/2022] [Accepted: 01/03/2023] [Indexed: 01/11/2023] Open
Abstract
High infiltration of tumor-associated macrophages (TAMs), which contribute to the progression of several cancer types, is correlated with poor prognosis of esophageal squamous cell carcinoma (ESCC). In addition to the previously reported increase in migration and invasion, ESCC cells co-cultured directly with macrophages exhibited enhanced survival and growth. Furthermore, interleukin-related molecules are associated with ESCC; however, the precise mechanism underlying this association is unclear. Therefore, we explored the role of interleukin-related molecules in ESCC progression. A cDNA microarray analysis of monocultured and co-cultured ESCC cells revealed that the interleukin 7 receptor (IL-7R) was upregulated in ESCC cells co-cultured with macrophages. Overexpression of IL-7R promoted the survival and growth of ESCC cells by activating the Akt and Erk1/2 signaling pathways. The IL-7/IL-7R axis also contributed to the promotion of ESCC cell migration via the Akt and Erk1/2 signaling pathways. Furthermore, immunohistochemistry showed that ESCC patients with high IL-7R expression in cancer nests exhibited a trend toward poor prognosis in terms of disease-free survival, and showed significant correlation with increased numbers of infiltrating macrophages and cancer-associated fibroblasts. Therefore, IL-7R, which is upregulated when directly co-cultured with macrophages, may contribute to ESCC progression by promoting the development of various malignant phenotypes in cancer cells.
Collapse
Affiliation(s)
- Yu Kitamura
- Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
- Division of Gastro-Intestinal Surgery, Department of Surgery, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| | - Yu-ichiro Koma
- Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| | - Kohei Tanigawa
- Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
- Division of Gastro-Intestinal Surgery, Department of Surgery, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| | - Shuichi Tsukamoto
- Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| | - Yuki Azumi
- Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
- Division of Gastro-Intestinal Surgery, Department of Surgery, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| | - Shoji Miyako
- Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
- Division of Gastro-Intestinal Surgery, Department of Surgery, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| | - Satoshi Urakami
- Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
- Division of Gastroenterology, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| | - Takayuki Kodama
- Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| | - Mari Nishio
- Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| | - Manabu Shigeoka
- Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| | - Yoshihiro Kakeji
- Division of Gastro-Intestinal Surgery, Department of Surgery, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| | - Hiroshi Yokozaki
- Division of Pathology, Department of Pathology, Kobe University Graduate School of Medicine, Kobe 650-0017, Japan
| |
Collapse
|
|
81
|
Fu C, Feng S, Wang S, Su X. Development and validation of a prognostic model for esophageal carcinoma based on immune microenvironment using system bioinformatics. Cancer Med 2023; 12:2089-2103. [PMID: 35771026 PMCID: PMC9883539 DOI: 10.1002/cam4.4985] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/09/2021] [Revised: 05/02/2022] [Accepted: 06/11/2022] [Indexed: 02/02/2023] Open
Abstract
Esophageal cancer (EC) is an aggressive malignancy that accounts for numerous cancer-related deaths worldwide. The multimodal combination therapy approach can be potentially used to treat EC effectively. However, distinct biomarker of significant specificity are still needed to develop individualized treatment strategies and provide accurate prognostic predictions. Therefore, we aimed to investigate the associated genes subtypes identified were, IFN-γDominant, Inflammatory, Lymphocyte Depleted, etc. and construct a risk model based on these genes to predict the overall survival (OS) of patients suffering from EC. Three immune subtypes were defined in the Cancer Genome Atlas (TCGA) cohort with different tumor microenvironment (TME) and clinical outcomes based on radio-differentiated immune genes. Subsequently, a risk model of immune characteristics included the immune cell infiltration levels and pathway activity was developed based on the genomic changes between the subtypes. In the TCGA dataset, as well as in subgroup analysis with different stages, gender, age, and pathological type, a high-risk score was identified as an adverse factor for OS using the method of the univariate Cox regression analysis and tROC analysis. Furthermore, it was observed that the high-risk group was characterized by depleted immunophenotype, active cell metabolism, and a high tumor mutation burden (TMB). The low-risk group was characterized by high TME abundance and active immune function. Differences in the biological genotypes may account for the differences in the prognosis and treatment response. Extensive research was carried out, and the results revealed that the low-risk group exhibited a significant level of therapeutic advantage in the field of immunotherapy. A risk model was developed based on the immune characteristics. It can be used to optimize risk stratification for patients suffering from EC. The results can potentially help provide new perspectives on treatment methods.
Collapse
Affiliation(s)
- Chenchun Fu
- Department of OncologyZhongda Hospital, Southeast UniversityNanjingChina
| | - Shicheng Feng
- Department of OncologyZhongda Hospital, Southeast UniversityNanjingChina
| | - Sheng Wang
- Department of OncologyZhongda Hospital, Southeast UniversityNanjingChina
| | - Xiangyu Su
- Department of OncologyZhongda Hospital, Southeast UniversityNanjingChina
| |
Collapse
|
|
82
|
Zandieh MA, Farahani MH, Rajabi R, Avval ST, Karimi K, Rahmanian P, Razzazan M, Javanshir S, Mirzaei S, Paskeh MDA, Salimimoghadam S, Hushmandi K, Taheriazam A, Pandey V, Hashemi M. Epigenetic regulation of autophagy by non-coding RNAs in gastrointestinal tumors: Biological functions and therapeutic perspectives. Pharmacol Res 2023; 187:106582. [PMID: 36436707 DOI: 10.1016/j.phrs.2022.106582] [Citation(s) in RCA: 15] [Impact Index Per Article: 7.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 10/25/2022] [Revised: 11/21/2022] [Accepted: 11/23/2022] [Indexed: 11/26/2022]
Abstract
Cancer is the manifestation of changes and mutations in genetic and epigenetic levels. Non-coding RNAs (ncRNAs) are commonly dysregulated in disease pathogenesis, and their role in cancer has been well-documented. The ncRNAs regulate various molecular pathways and mechanisms in cancer that can lead to induction/inhibition of carcinogenesis. Autophagy is a molecular "self-digestion" mechanism its function can be pro-survival or pro-death in tumor cells. The aim of the present review is to evaluate the role of ncRNAs in regulating autophagy in gastrointestinal tumors. The role of the ncRNA/autophagy axis in affecting the progression of gastric, liver, colorectal, pancreatic, esophageal, and gallbladder cancers is investigated. Both ncRNAs and autophagy mechanisms can function as oncogenic or onco-suppressor and this interaction can determine the growth, invasion, and therapy response of gastrointestinal tumors. ncRNA/autophagy axis can reduce/increase the proliferation of gastrointestinal tumors via the glycolysis mechanism. Furthermore, related molecular pathways of metastasis, such as EMT and MMPs, are affected by the ncRNA/autophagy axis. The response of gastrointestinal tumors to chemotherapy and radiotherapy can be suppressed by pro-survival autophagy, and ncRNAs are essential regulators of this mechanism. miRNAs can regulate related genes and proteins of autophagy, such as ATGs and Beclin-1. Furthermore, lncRNAs and circRNAs down-regulate miRNA expression via sponging to modulate the autophagy mechanism. Moreover, anti-cancer agents can affect the expression level of ncRNAs regulating autophagy in gastrointestinal tumors. Therefore, translating these findings into clinics can improve the prognosis of patients.
Collapse
Affiliation(s)
- Mohammad Arad Zandieh
- Department of Food Hygiene and Quality Control, Division of Epidemiology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
| | - Melika Heydari Farahani
- Faculty of Veterinary Medicine, Islamic Azad University, Shahr-e kord Branch, Chaharmahal and Bakhtiari, Iran
| | - Romina Rajabi
- Faculty of Veterinary Medicine, Islamic Azad University, Science and Research Branch, Tehran, Iran
| | | | - Kimia Karimi
- Faculty of Veterinary Medicine, Islamic Azad University, Science and Research Branch, Tehran, Iran
| | - Parham Rahmanian
- Faculty of Veterinary Medicine, Islamic Azad University, Science and Research Branch, Tehran, Iran
| | - Mehrnaz Razzazan
- Medical Student, Student Research Committee, Golestan University of Medical Sciences, Gorgan, Iran
| | - Salar Javanshir
- Young Researchers and Elite Club, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
| | - Sepideh Mirzaei
- Department of Biology, Faculty of Science, Islamic Azad University, Science and Research Branch, Tehran, Iran
| | - Mahshid Deldar Abad Paskeh
- Farhikhtegan Medical Convergence Sciences Research Center, Farhikhtegan Hospital Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
| | - Shokooh Salimimoghadam
- Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran
| | - Kiavash Hushmandi
- Department of Food Hygiene and Quality Control, Division of Epidemiology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran.
| | - Afshin Taheriazam
- Farhikhtegan Medical Convergence Sciences Research Center, Farhikhtegan Hospital Tehran Medical Sciences, Islamic Azad University, Tehran, Iran; Department of Orthopedics, Faculty of medicine, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran.
| | - Vijay Pandey
- Precision Medicine and Healthcare Research Center, Tsinghua-Berkeley Shenzhen Institute, Tsinghua University, Shenzhen 518055, Guangdong, China; Institute of Biopharmaceutical and Health Engineering, Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen 518055, China.
| | - Mehrdad Hashemi
- Farhikhtegan Medical Convergence Sciences Research Center, Farhikhtegan Hospital Tehran Medical Sciences, Islamic Azad University, Tehran, Iran; Department of Genetics, Faculty of Advanced Science and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran.
| |
Collapse
|
|
83
|
Han J, Hayashi S, Takahashi RU, Hirohata R, Kurokawa T, Tashiro M, Yamamoto Y, Okada M, Tahara H. Leukocyte Telomeric G-Tail Length Shortening Is Associated with Esophageal Cancer Recurrence. J Clin Med 2022; 11:jcm11247385. [PMID: 36556001 PMCID: PMC9784295 DOI: 10.3390/jcm11247385] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/31/2022] [Revised: 12/08/2022] [Accepted: 12/09/2022] [Indexed: 12/14/2022] Open
Abstract
Despite significant advances in therapeutics for esophageal cancer (ESC) in the past decade, it remains the sixth most fatal malignancy, with a poor 5-year survival rate (approximately 10%). There is an urgent need to improve the timely diagnosis to aid the prediction of the therapeutic response and prognosis of patients with ESC. The telomeric G-tail plays an important role in the chromosome protection. However, aging and age-related diseases lead to its shortening. Therefore, the G-tail length has been proposed as a novel potential biomarker. In the present study, to examine the possibility of G-tail shortening in patients with ESC, we measured the leukocyte telomere length (LTL) and the G-tail length using a hybridization protection assay in 147 patients with ESC and 170 age-matched healthy controls. We found that the G-tail length in patients with ESC was shorter than that in the healthy controls (p = 0.02), while the LTL shortening was not correlated with the ESC incidence and recurrence. Our results suggest that the G-tail length reflects the physiological status of patients with ESC and is a promising biomarker for the diagnosis and prognosis of ESC.
Collapse
Affiliation(s)
- Jiayan Han
- Department of Cellular and Molecular Biology, Graduate School of Biomedical and Health Sciences, Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-8553, Japan
| | - Soichiro Hayashi
- Department of Cellular and Molecular Biology, Graduate School of Biomedical and Health Sciences, Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-8553, Japan
| | - Ryou-u Takahashi
- Department of Cellular and Molecular Biology, Graduate School of Biomedical and Health Sciences, Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-8553, Japan
| | - Ryosuke Hirohata
- Department of Surgical Oncology, Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-0037, Japan
| | - Tomoaki Kurokawa
- Department of Surgical Oncology, Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-0037, Japan
| | - Mizuki Tashiro
- Department of Cellular and Molecular Biology, Graduate School of Biomedical and Health Sciences, Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-8553, Japan
| | - Yuki Yamamoto
- Department of Cellular and Molecular Biology, Graduate School of Biomedical and Health Sciences, Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-8553, Japan
| | - Morihito Okada
- Department of Surgical Oncology, Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-0037, Japan
| | - Hidetoshi Tahara
- Department of Cellular and Molecular Biology, Graduate School of Biomedical and Health Sciences, Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-8553, Japan
- Correspondence: ; Tel.: +81-08-2257-5290 (ext. 5290)
| |
Collapse
|
|
84
|
Zhu M, Shi B, Li C, Xu S. TET3 governs malignant behaviors and unfavorable prognosis of esophageal squamous cell carcinoma by activating the PI3K/AKT/GSK3β/β-catenin pathway. Open Med (Wars) 2022; 17:1883-1895. [PMID: 36518116 PMCID: PMC9719370 DOI: 10.1515/med-2022-0601] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/21/2022] [Revised: 10/07/2022] [Accepted: 10/16/2022] [Indexed: 01/23/2025] Open
Abstract
Ten-eleven translocation 3 (TET3) participates in tumorigenesis and malignant transformation by mediating DNA demethylation and specific gene activation in malignances. This study aims to elucidate its molecular function and regulatory mechanism in esophageal squamous cell carcinoma (ESCC). Stable ESCC cells that infected with TET3 overexpression (OE) and knockdown lentiviral vector had been established. The biological behaviors and molecular mechanism of TET3 were demonstrated by cell biology experiments in vitro and in vivo. Tissues from patients with ESCC were used to demonstrate the clinical value of TET3. Our findings revealed that TET3 is highly expressed in ESCC tissues and related to poor prognosis of patients with ESCC. OE of TET3 presented a significant effect on proliferation, metastatic potential, and spheroid formation of ESCC cells by activating the PI3K/AKT/GSK3β/β-catenin axis. Knockdown of TET3 could remarkably reverse these malignant phenotypes. Patients with ESCC with high TET3 expression resulted in a shorter overall survival (OS) and disease-free survival. Based on the multivariate analysis, TET3 could be an independent favorable factor for predicting OS and recurrence. The high expression of TET3 not only aggravates malignant behaviors in vitro and in vivo but also becomes a novel biomarker for clinical monitoring and individualized precision treatment for patients with ESCC.
Collapse
Affiliation(s)
- Maoling Zhu
- Department of Gastroenterology, Yangpu Hospital, School of Medicine, Tongji University, Shanghai 200090, P.R. China
| | - Bowen Shi
- Department of Thoracic Surgery, Changhai Hospital, Navy Military Medical University, Shanghai 200438, China
| | - Chunguang Li
- Department of Thoracic Surgery, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai 200030, China
| | - Shuchang Xu
- Department of Gastroenterology, Tongji Institute of Digestive Disease, Tongji Hospital, School of Medicine, Tongji University, Shanghai 200065, P.R. China
| |
Collapse
|
|
85
|
Mohammadi E, Aliarab A, Babaei G, Habibi NK, Jafari SM, Mir SM, Memar MY. MicroRNAs in esophageal squamous cell carcinoma: Application in prognosis, diagnosis, and drug delivery. Pathol Res Pract 2022; 240:154196. [PMID: 36356334 DOI: 10.1016/j.prp.2022.154196] [Citation(s) in RCA: 7] [Impact Index Per Article: 2.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 09/02/2022] [Revised: 10/26/2022] [Accepted: 10/29/2022] [Indexed: 11/09/2022]
Abstract
MicroRNAs (miRNAs) play a vital role in various cell biology processes, including cancer formation. These small non-coding RNAs could function as diagnostic and prognostic markers. They may involve esophageal squamous cell carcinoma (ESCC) and distinctive miRNA expression profiles; they are also known as therapeutic targets in human diseases. Therefore, in this study, the function of miRNAs was reviewed regarding the prognosis and diagnosis of ESCC. The changes in miRNAs before and after cancer therapy and the effects of miRNAs on chemo-susceptibility patterns were also investigated. MiRNA delivery systems in ESCC were also highlighted, providing a perspective on how these systems can improve miRNA efficiency.
Collapse
Affiliation(s)
- Elahe Mohammadi
- Department of Nutrition, Khalkhal University of Medical Sciences, Khalkhal, Iran
| | - Azadeh Aliarab
- Department of Clinical Biochemistry, School of Medicine, Tarbiat Modares University, Tehran, Iran
| | - Ghader Babaei
- Department of Clinical Biochemistry, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
| | - Nasim Kouhi Habibi
- Cellular and Molecular Biology Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran
| | - Seyyed Mehdi Jafari
- Metabolic Disorders Research Center, Golestan University of Medical Sciences, Gorgan, Iran.
| | - Seyed Mostafa Mir
- Metabolic Disorders Research Center, Golestan University of Medical Sciences, Gorgan, Iran.
| | - Mohammad Yousef Memar
- Infectious and Tropical Diseases Research Centre, Tabriz University of Medical Sciences, Tabriz, Iran.
| |
Collapse
|
|
86
|
Haghi B, Saghaeian Jazi M, Khosravi A, Jafari SM, Asadi J. SOX2OT lncRNA Inhibition Suppresses the Stemness Characteristics of Esophageal Tumorspheres. Noncoding RNA 2022; 8:ncrna8060080. [PMID: 36548179 PMCID: PMC9782980 DOI: 10.3390/ncrna8060080] [Citation(s) in RCA: 1] [Impact Index Per Article: 0.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/16/2022] [Revised: 11/15/2022] [Accepted: 11/23/2022] [Indexed: 11/29/2022] Open
Abstract
BACKGROUND SOX2OT is a novel cancer associated long non-coding RNA (LncRNA) with higher expression in variable tumor tissues, including esophageal squamous cell carcinoma (ESCC). It also plays an important function in embryonic neuronal development. Regarding its function in both stemness and carcinogenesis, here, we aimed to investigate its expression and function in tumorspheres of the esophagus using the RNAi method. MATERIAL & METHODS Two esophageal squamous cancer cells (ESCC): KYSE30 and YM1 cells were used for sphere enrichment. Cells were transfected with SOX2OT targeting and control siRNA. The size and the number of spheres were measured using light microscopy. Gene expression of the pluripotency genes was measured by qRT-PCR and docetaxel chemoresistance was assessed by MTS viability assay. RESULTS Our findings showed that ESCC tumorspheres overexpress SOX2OT gene along with other stemness genes (SOX2, OCT4A, and Nanog) compared to their original cancer cells. RNAi experiments indicated that SOX2OT knockdown can suppress the stemness-related gene expression, sphere formation ability (both size and number), and docetaxel resistance as three of the main cancer stem cell characteristics of tumorspheres. CONCLUSION Altogether our results showed the regulatory role of SOX2OT in pluripotency and stemness in ESCC tumorspheres. Our results suggest a potential application of SOX2OT inhibition in combination with docetaxel for ESCC inhibition in vitro.
Collapse
Affiliation(s)
- Boshra Haghi
- Metabolic Disorders Research Center, Golestan University of Medical Sciences, Gorgan 4934174515, Iran
| | - Marie Saghaeian Jazi
- Metabolic Disorders Research Center, Golestan University of Medical Sciences, Gorgan 4934174515, Iran
- Stem Cell Research Center, Golestan University of Medical Sciences, Gorgan 4934174515, Iran
- Correspondence: (M.S.J.); (J.A.)
| | - Ayyoob Khosravi
- Stem Cell Research Center, Golestan University of Medical Sciences, Gorgan 4934174515, Iran
- Department of Molecular Medicine, Faculty of Advanced Medical Technologies Golestan, University of Medical Sciences, Gorgan 4934174516, Iran
| | - Seyyed Mehdi Jafari
- Metabolic Disorders Research Center, Golestan University of Medical Sciences, Gorgan 4934174515, Iran
| | - Jahanbakhsh Asadi
- Metabolic Disorders Research Center, Golestan University of Medical Sciences, Gorgan 4934174515, Iran
- Correspondence: (M.S.J.); (J.A.)
| |
Collapse
|
|
87
|
Tsai MC, Chou YC, Lee YK, Hsu WL, Tang CS, Chen SY, Huang SP, Chen YC, Lee JM. Secular Trends in Incidence of Esophageal Cancer in Taiwan from 1985 to 2019: An Age-Period-Cohort Analysis. Cancers (Basel) 2022; 14:cancers14235844. [PMID: 36497327 PMCID: PMC9741308 DOI: 10.3390/cancers14235844] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/17/2022] [Revised: 11/19/2022] [Accepted: 11/23/2022] [Indexed: 11/29/2022] Open
Abstract
In Taiwan, the age-standardized incidence of EC, especially esophageal squamous cell carcinoma (ESCC), has increased substantially during the past thirty years. We described the incidence trends of EC from 1985−2019 by an average annual percentage change (AAPC) and age-period-cohort model by using Taiwan Cancer Registry data. Age-period-cohort modeling was used to estimate the period and cohort effects of ESCC and esophageal adenocarcinoma (EAC). The Spearman’s correlation coefficient was used to analyze the correlation between age-adjusted incidence rates of EC and the prevalence of risk factors from national surveys. The results showed the incidence rate of ESCC in men (AAPC = 4.2, 95% CI = 3.1−5.4, p < 0.001) increased prominently from 1985−1989 to 2015−2019 while that of EAC in men (AAPC = 1.2, 95% CI = 0.9−1.5, p < 0.001) and ESCC in women (AAPC = 1.7, 95% CI = 1.4−2.1, p < 0.001) increased to a lesser degree. Increased period effects were observed in ESCC in men, ESCC in women, and EAC in men. High correlations were found between the risk factors and the increased birth-cohort effects of ESCC (p < 0.05). To conclude, the incidence of ESCC in both sex and EAC in men increased with statistical significance in recent decades. The increased prevalence of risk factors from approximately 1970−1995 could explain the increased cohort effects of ESCC.
Collapse
Affiliation(s)
- Min-Chen Tsai
- School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei City 242008, Taiwan
| | - Yu-Ching Chou
- School of Public Health, National Defense Medical Center, Taipei 11490, Taiwan
| | - Yu-Kwang Lee
- Division of General Surgery, Department of Surgery, National Taiwan University Hospital, Taipei 100225, Taiwan
| | - Wan-Lun Hsu
- Master Program of Big Data in Biomedicine, College of Medicine, Fu Jen Catholic University, New Taipei City 242008, Taiwan
- Data Science Center, College of Medicine, Fu Jen Catholic University, New Taipei City 242008, Taiwan
| | - Chin-Sheng Tang
- Department of Public Health, College of Medicine, Fu Jen Catholic University, New Taipei City 242008, Taiwan
| | - Shiow-Ying Chen
- Department of Medical Research, Fu Jen Catholic University Hospital, New Taipei City 24352, Taiwan
| | - Shih-Pei Huang
- Department of Medical Education & Bioethics, Graduate Institute of Medical Education & Bioethics, National Taiwan University College of Medicine, Taipei 10051, Taiwan
| | - Yong-Chen Chen
- Master Program of Big Data in Biomedicine, College of Medicine, Fu Jen Catholic University, New Taipei City 242008, Taiwan
- Data Science Center, College of Medicine, Fu Jen Catholic University, New Taipei City 242008, Taiwan
- Correspondence: ; Tel.: +886-2-29056221
| | - Jang-Ming Lee
- Department of Surgery, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei 100225, Taiwan
| |
Collapse
|
|
88
|
Lin X, Tu M, Zhang Y, Zhuang W, Cai L, Zhang L, Yu L, Zhang Z, Huang Y. Aberrant NSG1 Expression Promotes Esophageal Squamous Cell Carcinoma Cell EMT by the Activation of ERK Signaling Pathway. Dig Dis Sci 2022; 68:1847-1857. [PMID: 36396779 DOI: 10.1007/s10620-022-07748-6] [Citation(s) in RCA: 5] [Impact Index Per Article: 1.7] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 02/24/2022] [Accepted: 10/26/2022] [Indexed: 11/19/2022]
Abstract
BACKGROUND Neuron-specific gene family member 1 (NSG1) is a 21 kDa endosomal protein that is specifically expressed in neurons. AIMS This study was to explore the expression of NSG1 and possible mechanism in Esophageal Squamous Cell Carcinoma (ESCC). METHODS The Cancer Genome Atlas (TCGA) database was consulted to analyze the expression of NSG1 in ESCC. Immunohistochemistry (IHC) staining was used to evaluate NSG1 expression in ESCC cancerous tissues and matched paracancerous tissues. The CCK-8 assay, wound-healing assay, and transwell assay were used to detect the cell viability, migration, and invasion of ESCC cells. Western blot was used to assay epithelial-mesenchymal transition (EMT)-related proteins and ERK signaling pathway protein expression. RESULTS The results showed that the expression of NSG1 in ESCC cancerous tissues was higher than noncancerous tissues. Compared with negative control (NC) group, cell viability, migration. and invasion significantly increased, the expression of p-ERK in ERK signaling pathway was significantly upregulated, the expressions of mesenchymal marker Vimentin and EMT-related transcription factors including snail and slug were significantly upregulated, and the expression of epithelial marker E-cadherin was significantly downregulated in KYSE-150 cells with NSG1 overexpression. However, these effects were reversed by the ERK signaling pathway inhibitor U0126. On the other hand, TE-1 cells with NSG1 knockdown had the changes contrary to that in KYSE-150 cells with NSG1 overexpression. CONCLUSION NSG1 plays a potential carcinogenic role on the occurrence and progression of ESCC, and aberrant NSG1 expression might promote ESCC cell EMT by the activation of ERK signaling pathway.
Collapse
Affiliation(s)
- Xiaoqing Lin
- Shengli Clinical Medical College of Fujian Medical University, Fuzhou, 350001, Fujian, China.,Department of Clinical Laboratory, Fujian Provincial Hospital, Fuzhou, 350001, Fujian, China
| | - Mingshu Tu
- Shengli Clinical Medical College of Fujian Medical University, Fuzhou, 350001, Fujian, China.,Department of Clinical Laboratory, Fujian Provincial Hospital, Fuzhou, 350001, Fujian, China
| | - Yi Zhang
- Shengli Clinical Medical College of Fujian Medical University, Fuzhou, 350001, Fujian, China.,Department of Clinical Laboratory, Fujian Provincial Hospital, Fuzhou, 350001, Fujian, China
| | - Wanzhen Zhuang
- Shengli Clinical Medical College of Fujian Medical University, Fuzhou, 350001, Fujian, China.,Department of Clinical Laboratory, Fujian Provincial Hospital, Fuzhou, 350001, Fujian, China
| | - Liqing Cai
- Shengli Clinical Medical College of Fujian Medical University, Fuzhou, 350001, Fujian, China.,Department of Clinical Laboratory, Fujian Provincial Hospital, Fuzhou, 350001, Fujian, China
| | - Liangming Zhang
- Shengli Clinical Medical College of Fujian Medical University, Fuzhou, 350001, Fujian, China.,Department of Clinical Laboratory, Fujian Provincial Hospital, Fuzhou, 350001, Fujian, China
| | - Lili Yu
- Shengli Clinical Medical College of Fujian Medical University, Fuzhou, 350001, Fujian, China.,Department of Clinical Laboratory, Fujian Provincial Hospital, Fuzhou, 350001, Fujian, China
| | - Zhenlong Zhang
- Shengli Clinical Medical College of Fujian Medical University, Fuzhou, 350001, Fujian, China.,Department of Thoracic Surgery, Fujian Provincial Hospital, Fuzhou, 350001, Fujian, China
| | - Yi Huang
- Shengli Clinical Medical College of Fujian Medical University, Fuzhou, 350001, Fujian, China. .,Department of Clinical Laboratory, Fujian Provincial Hospital, Fuzhou, 350001, Fujian, China. .,Central Laboratory, Center for Experimental Research in Clinical Medicine, Fujian Provincial Hospital, Fuzhou, 350001, Fujian, China.
| |
Collapse
|
|
89
|
INPP5A/HLA-G1/IL-10/MMP-21 Axis in Progression of Esophageal Squamous Cell Carcinoma. IRANIAN BIOMEDICAL JOURNAL 2022; 26:440-53. [PMID: 36437782 PMCID: PMC9841225 DOI: 10.52547/ibj.3716] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Figures] [Subscribe] [Scholar Register] [Indexed: 12/14/2022]
Abstract
Background Background: Type I inositol polyphosphate-5-phosphatase A (INPP5A) is involved in different cellular events, including cell proliferation. Since INPP5A, HLAG1, IL-10, and matrix metalloproteinases (MMP)-21 genes play fundamental roles in esophageal squamous cell carcinoma (ESCC) tumorigenesis, we aimed in this study to clarify the possible interplay of these genes and explore the potential of these chemistries as a predictor marker for diagnosis in ESCC disease. Methods Methods: Gene expression analysis of INPP5A, HLAG-1, IL-10, and MMP-21 was performed using relative comparative real-time PCR in 56 ESCCs compared to their margin normal tissues. Immunohistochemical staining was accomplished for INPP5A in ESCCs. Analysis of ROC curves and the AUC were applied to evaluate the diagnostic capability of the candidate genes. Results Results: High levels of HLA-G1, MMP-21, and IL-10 were detected in nearly 23.2%, 62.5%, and 53.5% of ESCCs compared to the normal tissues, respectively, whereas INPP5A underexpression was detected in 19.6% of ESCCs, which all tested genes indicated significant correlations with each other. The protein expression level of INPP5A in ESCC tissues was significantly lower than that of the non-tumor esophageal tissues (p = 0.001). Interestingly, the concomitant expression of the INPP5A/HLA-G1, INPP5A/MMP-21, INPP5A/IL-10, HLA-G1/MMP-21, HLA-G1/IL-10, and MMP-21/IL-10 was significantly correlated with several clinicopathological variables. INPP5A, HLA-G1, MMP-21, and IL-10 showed to be the most appropriate candidates to discriminate tumor/non-tumor groups due to the total AUCs of all combinations (>60%). Conclusion Conclusion: Our results represent a new regulatory axis containing INPP5A/HLAG-1/IL-10/MMP-21 markers in ESCC development and may provide novel insight into the mechanism of immune evasion mediated
by the INPP5A/HLAG-1/IL-10/MMP-21 regulatory network in the disease.
Collapse
|
|
90
|
Overall Survival for Esophageal Squamous Cell Carcinoma with Multiple Primary Cancers after Curative Esophagectomy-A Retrospective Single-Institution Study. Cancers (Basel) 2022; 14:cancers14215263. [PMID: 36358682 PMCID: PMC9658684 DOI: 10.3390/cancers14215263] [Citation(s) in RCA: 1] [Impact Index Per Article: 0.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/19/2022] [Revised: 10/03/2022] [Accepted: 10/25/2022] [Indexed: 11/17/2022] Open
Abstract
Background: Advances in surgical techniques and treatment modalities have improved the outcomes of esophageal cancer, yet difficult decision making for physicians while encountering multiple primary cancers (MPCs) continues to exist. The aim of this study was to evaluate long-term survival for esophageal squamous cell carcinoma (SCC) associated with MPCs. Methods: Data from 544 patients with esophageal SCC who underwent surgery between 2005 and 2017 were reviewed to identify the presence of simultaneous or metachronous primary cancers. The prognostic factors for overall survival (OS) were analyzed. Results: Three hundred and ninety-seven patients after curative esophagectomy were included, with a median observation time of 44.2 months (range 2.6−178.6 months). Out of 52 patients (13.1%) with antecedent/synchronous cancers and 296 patients without MPCs (control group), 49 patients (12.3%) developed subsequent cancers after surgery. The most common site of other primary cancers was the head and neck (69/101; 68.3%), which showed no inferiority in OS. Sex and advanced clinical stage (III/IV) were independent risk factors (p = 0.031 and p < 0.001, respectively). Conclusion: Once curative esophagectomy can be achieved, surgery should be selected as a potential therapeutic approach if indicated, even with antecedent/synchronous MPCs. Subsequent primary cancers were often observed in esophageal SCC, and optimal surveillance planning was recommended.
Collapse
|
|
91
|
Toda S, Hatta W, Asanuma K, Asano N, Ono Y, Abe H, Ogata Y, Saito M, Kanno T, Jin X, Uno K, Koike T, Imatani A, Hamada S, Nakamura T, Nakaya N, Masamune A. Decreased Expression of NRF2 Target Genes after Alcohol Exposure in the Background Esophageal Mucosa of Patients with Esophageal Squamous Cell Carcinoma. TOHOKU J EXP MED 2022; 258:195-206. [PMID: 36070895 DOI: 10.1620/tjem.2022.j077] [Citation(s) in RCA: 2] [Impact Index Per Article: 0.7] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 11/18/2022]
Abstract
Patients with esophageal squamous cell carcinoma (ESCC) might have a specific mechanism for the carcinogenesis by alcohol consumption in the background esophageal mucosa, and nuclear factor erythroid 2-related factor 2 (NRF2), which plays a protective role against esophageal carcinogenesis, and barrier dysfunction might be associated with this phenomenon. This study aimed to confirm this hypothesis. Twenty patients with superficial ESCCs (ESCC patients) and 20 age- and sex-matched patients without ESCC (non-ESCC patients) were enrolled. Biopsy samples were obtained from non-neoplastic esophageal mucosa: one for histological evaluation, one for quantitative real-time polymerase chain reaction (PCR), and two for the mini-Ussing chamber system to measure transepithelial electrical resistance (TEER) and, thereafter, for PCR. The TEER after acetaldehyde or both acetaldehyde and ethanol exposure did not differ significantly between ESCC and non-ESCC patients. Unlike non-ESCC patients, mRNA levels of NRF2 target genes and claudin4 in ESCC patients tended to decrease after the exposure, with a significant difference between no exposure and both acetaldehyde and ethanol exposure in NRF2 target genes (p < 0.05). Furthermore, in ESCC patients, the decreased tendency of mRNA levels of NRF2 target genes after the exposure was more pronounced in high-risk states, such as aldehyde dehydrogenase 2 (ALDH2) Lys alleles (Glu/Lys + Lys/Lys), Lugol-voiding lesion grade C, and drinking history. In conclusion, the protective role of NRF2 against carcinogenesis from alcohol exposure might be disrupted in the background esophageal mucosa of ESCC patients, which might lead to a high incidence of metachronous ESCC.
Collapse
Affiliation(s)
- Shusuke Toda
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Waku Hatta
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Kiyotaka Asanuma
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Naoki Asano
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Yoshitaka Ono
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Hiroko Abe
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Yohei Ogata
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Masahiro Saito
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Takeshi Kanno
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Xiaoyi Jin
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Kaname Uno
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Tomoyuki Koike
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Akira Imatani
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Shin Hamada
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| | - Tomohiro Nakamura
- Department of Health Record Informatics, Tohoku Medical Megabank Organization, Tohoku University
| | - Naoki Nakaya
- Department of Preventive Medicine and Epidemiology, Tohoku Medical Megabank Organization, Tohoku University
| | - Atsushi Masamune
- Division of Gastroenterology, Tohoku University Graduate School of Medicine
| |
Collapse
|
|
92
|
Chen H, Sun G, Han Z, Wang H, Li J, Ye H, Song C, Zhang J, Wang P. Anti-CXCL8 Autoantibody: A Potential Diagnostic Biomarker for Esophageal Squamous Cell Carcinoma. MEDICINA (KAUNAS, LITHUANIA) 2022; 58:medicina58101480. [PMID: 36295640 PMCID: PMC9607113 DOI: 10.3390/medicina58101480] [Citation(s) in RCA: 2] [Impact Index Per Article: 0.7] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Download PDF] [Figures] [Subscribe] [Scholar Register] [Received: 07/23/2022] [Revised: 09/15/2022] [Accepted: 10/13/2022] [Indexed: 11/07/2022]
Abstract
Background and Objectives: Esophageal squamous cell carcinoma (ESCC) is one of the most common malignancies. Anti-tumor associated antigen autoantibodies (TAAbs) can be used as biomarkers for tumor detection. The aim of this study was to identify a reliable TAAb as the diagnostic marker for ESCC. Materials and Methods: The Cancer Genome Atlas (TCGA) database was used to screen candidate genes. The mRNA expression of the key gene was then verified by micro array dataset GSE44021 from the Gene Expression Omnibus (GEO) database and the diag nostic value of the corresponding autoantibody to the key gene in ESCC was detected by enzyme-linked im muno sorbent assay (ELISA). Results: CXCL8 was identified as the key gene. The dataset GSE44021 showed that CXCL8 mRNA expression was prominently over-expressed in ESCC tissues compared with normal tissues. ELISA results showed that the level of anti-CXCL8 autoantibody in ESCC patients was significantly higher than in normal controls and the receiver operating char ac teristic (ROC) curve indicated that anti-CXCL8 autoantibody could discriminate ESCC patients from normal controls, with the area under the ROC curve (AUC) for the verification cohort, and the validation cohort were 0.713 and 0.751, respectively. Conclusions: Our study illustrated that anti-CXCL8 autoantibody had good diagnostic value, and may become a candidate biomarker for ESCC.
Collapse
Affiliation(s)
- Huili Chen
- Department of Epidemiology and Statistics, College of Public Health, Zhengzhou University, Zhengzhou 450001, China
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou 450052, China
| | - Guiying Sun
- Department of Epidemiology and Statistics, College of Public Health, Zhengzhou University, Zhengzhou 450001, China
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou 450052, China
| | - Zhuo Han
- Department of Epidemiology and Statistics, College of Public Health, Zhengzhou University, Zhengzhou 450001, China
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou 450052, China
| | - Huimin Wang
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou 450052, China
- Henan Institute of Medical and Pharmaceutical Sciences, Zhengzhou University, Zhengzhou 450052, China
| | - Jiaxin Li
- Department of Epidemiology and Statistics, College of Public Health, Zhengzhou University, Zhengzhou 450001, China
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou 450052, China
| | - Hua Ye
- Department of Epidemiology and Statistics, College of Public Health, Zhengzhou University, Zhengzhou 450001, China
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou 450052, China
| | - Chunhua Song
- Department of Epidemiology and Statistics, College of Public Health, Zhengzhou University, Zhengzhou 450001, China
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou 450052, China
| | - Jianying Zhang
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou 450052, China
- Henan Institute of Medical and Pharmaceutical Sciences, Zhengzhou University, Zhengzhou 450052, China
| | - Peng Wang
- Department of Epidemiology and Statistics, College of Public Health, Zhengzhou University, Zhengzhou 450001, China
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou 450052, China
- Correspondence: ; Tel.: +86-0371-67781453
| |
Collapse
|
|
93
|
Zhou R, Huang C, Luo Z, Wang T. The Association between the Risk of Esophageal Cancer and Type 2 Diabetes Mellitus: An Updated Meta-Analysis. BIOMED RESEARCH INTERNATIONAL 2022; 2022:8129771. [PMID: 36277883 PMCID: PMC9584674 DOI: 10.1155/2022/8129771] [Citation(s) in RCA: 1] [Impact Index Per Article: 0.3] [Reference Citation Analysis] [Abstract] [MESH Headings] [Track Full Text] [Download PDF] [Figures] [Subscribe] [Scholar Register] [Received: 07/18/2022] [Revised: 08/15/2022] [Accepted: 09/20/2022] [Indexed: 12/24/2022]
Abstract
Background A large amount of publications had reported the association between incidence of esophageal cancer (EC) and type 2 diabetes mellitus (T2DM) in the past decade. However, those papers' results are inconsistent on relationships between T2DM the incidence of EC. Therefore, the objective of this meta-analysis was to determine the relationship between T2DM and the risk of EC (including 2 histological types, esophageal adenocarcinoma [EADC] and esophageal squamous cell carcinoma [ESCC]). Method We finally extracted 19 articles though Pubmed, Embased, and Cochrane library. Those identify extraction date including 14,312 cases and 24,959,067 control records and then mixed the relative risks (RRs) and corresponding 95% confidence intervals (95%CIs) through STATA. Results We observed that there are significantly positive correlation between T2DM and EC risk (RR = 1.28, 95% CI: 1.05-1.57, P = 0.015).Also, our study showed positive correlation between T2DM and EADC (esophageal adenocarcinoma) risk (RR = 1.28, 95% CI: 1.05-1.57, P < 0.001). What's more, subgroup analysis based on ethnicity represented the Caucasian is more susceptible to EC (RR = 1.28 ,95% CI: 1.10-1.49, P = 0.001). Conclusion Those results offer a recent epidemiological and integrated evidence to ascertain the correlations between T2DM and incidence of EC. Those results take public health implications on preventing T2DM and then depress the occurrence of EC. Our study also provides referenced information for the prevention. However, some data is still insufficient, and more research should be carried out.
Collapse
Affiliation(s)
- Runquan Zhou
- Department Of Thoracic Surgery, The Third Affiliated Hospital of Chongqing Medical University, Chongqing 401120, China
| | - Chenglu Huang
- Department of Thoracic Surgery, Chongqing University Cancer Hospital, No. 181 Hanyu Road, Shapingba District, Chongqing 400030, China
| | - Zhilin Luo
- Department Of Thoracic Surgery, The Third Affiliated Hospital of Chongqing Medical University, Chongqing 401120, China
| | - Tianhu Wang
- Department Of Thoracic Surgery, The Third Affiliated Hospital of Chongqing Medical University, Chongqing 401120, China
| |
Collapse
|
|
94
|
Ren L, Fang X, Shrestha SM, Ji Q, Ye H, Liang Y, Liu Y, Feng Y, Dong J, Shi R. LncRNA SNHG16 promotes development of oesophageal squamous cell carcinoma by interacting with EIF4A3 and modulating RhoU mRNA stability. Cell Mol Biol Lett 2022; 27:89. [PMID: 36221055 PMCID: PMC9552503 DOI: 10.1186/s11658-022-00386-w] [Citation(s) in RCA: 25] [Impact Index Per Article: 8.3] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/30/2022] [Accepted: 09/09/2022] [Indexed: 01/27/2023] Open
Abstract
BACKGROUND Numerous studies have revealed that long noncoding RNAs (lncRNAs) are closely related to the development of many diseases and carcinogenesis. However, their specific biological function and molecular mechanism in oesophageal squamous cell carcinoma (ESCC) remains unclear. METHODS RNA-Seq was performed to determine the differential expressions of lncRNAs in ESCC, and the level of SNHG16 expression was detected in ESCC and intraepithelial neoplasia (IEN) samples. In vitro and in vivo experiments were performed to explore the role of SNHG16 and the interaction of EIF4A3 and Ras homologue family member U (RhoU) signalling. RESULTS One hundred and seventy-five upregulated and 134 downregulated lncRNAs were identified by RNA-Seq. SNHG16 was highly expressed in ESCC and intraepithelial neoplasia (IEN) samples, and its expression level was correlated with tumour differentiation and T stage. Overexpression of SNHG16 can facilitate ESCC cell proliferation and metastasis. Mechanistically, we noticed that SNHG16 could bind RNA binding protein (RBP)-eukaryotic translation initiation factor (EIF4A3) and interact with it to form a complex. Importantly, the coalition of SNHG16 and EIF4A3 ultimately regulated Ras homologue family member U (RhoU). SNHG16 modulated RhoU expression by recruiting EIF4A3 to regulate the stability of RhoU mRNA. Knockdown of RhoU further alleviated the effect of the SNHG16 oncogene in ESCC cells. CONCLUSIONS The newly identified SNHG16-EIF4A3-RhoU signalling pathway directly coordinates the response in ESCC pathogenesis and suggests that SNHG16 is a promising target for potential ESCC treatment.
Collapse
Affiliation(s)
- Lihua Ren
- Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, 87 Dingjiaqiao Road, Nanjing, 210009, Jiangsu Province, People's Republic of China
| | - Xin Fang
- Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, 87 Dingjiaqiao Road, Nanjing, 210009, Jiangsu Province, People's Republic of China
| | - Sachin Mulmi Shrestha
- Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, 87 Dingjiaqiao Road, Nanjing, 210009, Jiangsu Province, People's Republic of China
| | - Qinghua Ji
- Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, 87 Dingjiaqiao Road, Nanjing, 210009, Jiangsu Province, People's Republic of China
| | - Hui Ye
- Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, 87 Dingjiaqiao Road, Nanjing, 210009, Jiangsu Province, People's Republic of China
| | - Yan Liang
- Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, 87 Dingjiaqiao Road, Nanjing, 210009, Jiangsu Province, People's Republic of China
| | - Yang Liu
- Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, 87 Dingjiaqiao Road, Nanjing, 210009, Jiangsu Province, People's Republic of China
| | - Yadong Feng
- Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, 87 Dingjiaqiao Road, Nanjing, 210009, Jiangsu Province, People's Republic of China
| | - Jingwu Dong
- Department of Gastroenterology, Xuyi County People's Hospital, Huaian, 211700, People's Republic of China
| | - Ruihua Shi
- Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, 87 Dingjiaqiao Road, Nanjing, 210009, Jiangsu Province, People's Republic of China.
| |
Collapse
|
|
95
|
Yang XL, Wang P, Ye H, Jiang M, Su YB, Peng XX, Li H, Zhang JY. Untargeted serum metabolomics reveals potential biomarkers and metabolic pathways associated with esophageal cancer. Front Oncol 2022; 12:938234. [PMID: 36176418 PMCID: PMC9513043 DOI: 10.3389/fonc.2022.938234] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 05/07/2022] [Accepted: 08/16/2022] [Indexed: 12/24/2022] Open
Abstract
Metabolomics has been reported as an efficient tool to screen biomarkers that are related to esophageal cancer. However, the metabolic biomarkers identifying malignant degrees and therapeutic efficacy are still largely unknown in the disease. Here, GC-MS-based metabolomics was used to understand metabolic alteration in 137 serum specimens from patients with esophageal cancer, which is approximately two- to fivefold as many plasma specimens as the previous reports. The elevated amino acid metabolism is in sharp contrast to the reduced carbohydrate as a characteristic feature of esophageal cancer. Comparative metabolomics showed that most metabolic differences were determined between the early stage (0–II) and the late stage (III and IV) among the 0–IV stages of esophageal cancer and between patients who received treatment and those who did not receive treatment. Glycine, serine, and threonine metabolism and glycine were identified as the potentially overlapped metabolic pathway and metabolite, respectively, in both disease progress and treatment effect. Glycine, fructose, ornithine, and threonine can be a potential array for the evaluation of disease prognosis and therapy in esophageal cancer. These results highlight the means of identifying previously unknown biomarkers related to esophageal cancer by a metabolomics approach.
Collapse
Affiliation(s)
- Xiao-li Yang
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou, China
- State Key Laboratory of Bio-Control, School of Life Sciences, Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Sun Yat-sen University, University City, Guangzhou, China
| | - Peng Wang
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou, China
- Henan Key Laboratory of Tumor Epidemiology and College of Public Health, Zhengzhou University, Zhengzhou, China
| | - Hua Ye
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou, China
- Henan Key Laboratory of Tumor Epidemiology and College of Public Health, Zhengzhou University, Zhengzhou, China
| | - Ming Jiang
- State Key Laboratory of Bio-Control, School of Life Sciences, Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Sun Yat-sen University, University City, Guangzhou, China
| | - Yu-bin Su
- Key Laboratory of Functional Protein Research of Guangdong Higher Education Institutes, Department of Biotechnology, College of Life Science and Technology, Jinan University, Guangzhou, China
| | - Xuan-xian Peng
- State Key Laboratory of Bio-Control, School of Life Sciences, Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Sun Yat-sen University, University City, Guangzhou, China
| | - Hui Li
- State Key Laboratory of Bio-Control, School of Life Sciences, Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Sun Yat-sen University, University City, Guangzhou, China
- *Correspondence: Jian-ying Zhang, ; Hui Li,
| | - Jian-ying Zhang
- State Key Laboratory of Esophageal Cancer Prevention & Treatment, Zhengzhou University, Zhengzhou, China
- Henan Academy of Medical and Pharmaceutical Sciences, Zhengzhou University, Zhengzhou, China
- *Correspondence: Jian-ying Zhang, ; Hui Li,
| |
Collapse
|
|
96
|
LOX and Its Methylation Impact Prognosis of Diseases and Correlate with TAM Infiltration in ESCA. JOURNAL OF ONCOLOGY 2022; 2022:5111237. [PMID: 36090891 PMCID: PMC9452977 DOI: 10.1155/2022/5111237] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Subscribe] [Scholar Register] [Received: 04/30/2022] [Accepted: 06/24/2022] [Indexed: 11/17/2022]
Abstract
Background ESCA is one of the digestive tract tumors with a high fatality. It is implicated in an intricate gene regulation process, but the pathogenesis remains ambiguous. Methods The study used the packages of Limma from R software to analyze DEGs of ESCA in the GEO database and TCGA database. We employed the DAVID website for enrichment analysis, and the string database constructed the PPI network. Hub genes were identified from ESCA DEGs with Cytoscape MCODE. We evaluated the clinical relevance of LOX expression and its DNA methylation in the cBioPortal database and explored the roles of LOX in ESCA immunity, especially immune cell infiltration levels and immune checkpoint expression, by immunedeconv package of R software. Conclusions The overexpression of LOX in ESCA is regulated by DNA hypomethylation; LOX overexpression or LOX hypomethylation can predict a worse prognosis in patients with ESCA. Besides, LOX may be involved in TIME regulation, promoting the infiltration levels and function of TAM. Hence, high LOX expression affected by DNA hypomethylation has an essential role in patients with ESCA, which may become an effective prognostic marker and therapeutic target.
Collapse
|
|
97
|
Li JJ, Xia XP, Wu LM, Zhu Z, Shi YN, Zhang XC, Xia YS, Lu GR. Cancer suppression by ferroptosis and its role in digestive system tumors. Shijie Huaren Xiaohua Zazhi 2022; 30:718-728. [DOI: 10.11569/wcjd.v30.i16.718] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Indexed: 02/07/2023] Open
Affiliation(s)
- Jia-Jia Li
- Department of Gastroenterology, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China
| | - Xuan-Ping Xia
- Department of Gastroenterology, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China
| | - Li-Min Wu
- Department of Gastroenterology, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China
| | - Zheng Zhu
- The Second Clinical Medical College of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China
| | - Yu-Ning Shi
- The Second Clinical Medical College of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China
| | - Xu-Chao Zhang
- The Second Clinical Medical College of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China
| | - Yu-Shan Xia
- The Second Clinical Medical College of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China
| | - Guang-Rong Lu
- Department of Gastroenterology, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China
| |
Collapse
|
|
98
|
Yu Y, Wu H, Qiu J, Ke D, Wu Y, Lin M, Liu T, Zheng Q, Zheng H, Yang J, Wang Z, Li H, Liu L, Yao Q, Li J, Cheng W, Chen X. A Nutrition-Related Factor-Based Risk Stratification for Exploring the Clinical Benefits in the Treatment of Patients With Locally Advanced Esophageal Squamous Cell Carcinoma Receiving Definitive Chemoradiotherapy: A Retrospective Cohort Study. Front Nutr 2022; 9:896847. [PMID: 35990358 PMCID: PMC9387592 DOI: 10.3389/fnut.2022.896847] [Citation(s) in RCA: 5] [Impact Index Per Article: 1.7] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/15/2022] [Accepted: 06/22/2022] [Indexed: 01/19/2023] Open
Abstract
Objective No study has reported the risk stratification of BMI and PNI in patients with locally advanced esophageal squamous cell carcinoma (ESCC) undergoing definitive chemoradiotherapy (dCRT). This study aimed to construct a risk stratification to guide the treatment of ESCC following dCRT. Methods A total of 1,068 patients with locally advanced ESCC who received dCRT were retrospectively analyzed. The impacts of clinicopathological factors on overall survival (OS) and progression-free survival (PFS) were analyzed. Besides, the novel prognostic indices of pre-therapeutic nutritional index (PTNI) and prognostic index (PI) were developed. Results The median follow-up period of OS and PFS were 22.9 and 17.4 months, respectively. The high body mass index (BMI) group had better 5-year OS and PFS (36.4 and 34.0%) than the low BMI group (18.8 and 17.2%). The high prognostic nutritional index (PNI) group also had better 5-year OS and PFS (33.4 and 30.9%) than the low PNI group (17.5 and 17.2%). Multivariate Cox regression analysis showed that BMI and PNI were independent prognostic factors for OS and PFS. Based on nutritional indices, patients were categorized into the low-risk (PTNI = 1), medium-risk (PTNI = 2), and high-risk (PTNI = 3) groups with 5-year OS rates of 38.5, 18.9, 17.5%, respectively (p < 0.001) and 5-year PFS rates of 35.8, 17.6, 16.8%, respectively (p < 0.001). Besides, we also constructed a prognostic index (PI) for OS and PFS which was calculated based on statistically significant factors for predicting OS and PFS. The results revealed that the high-risk group had worse OS and PFS than the low-risk group (p < 0.001). Finally, RCS analysis demonstrated a non-linear relationship between the PNI, BMI, and survival for patients with ESCC. The death hazard of PNI and BMI sharply decreased to 41.8 and 19.7. Conclusion The decreased pre-therapeutic BMI and PNI levels were associated with a worse survival outcome. BMI and PNI are readily available and can be used to stratify risk factors for locally advanced ESCC patients undergoing dCRT. The novel risk stratification may help to evaluate patients’ pre-therapeutic status and guide dCRT for locally advanced ESCC patients.
Collapse
Affiliation(s)
- Yilin Yu
- Department of Thoracic Surgery, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China.,College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Haishan Wu
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Jianjian Qiu
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Dongmei Ke
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Yahua Wu
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Mingqiang Lin
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Tianxiu Liu
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Qunhao Zheng
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Hongying Zheng
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Jun Yang
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Zhiping Wang
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Hui Li
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Lingyun Liu
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Qiwei Yao
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Jiancheng Li
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Wenfang Cheng
- College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China.,Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
| | - Xiaohui Chen
- Department of Thoracic Surgery, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China.,College of Clinical Medicine for Oncology, Fujian Medical University, Fuzhou, China.,The Graduate School, Fujian Medical University, Fuzhou, China
| |
Collapse
|
|
99
|
Xiao Y, Tang J, Yang D, Zhang B, Wu J, Wu Z, Liao Q, Wang H, Wang W, Su M. Long noncoding RNA LIPH-4 promotes esophageal squamous cell carcinoma progression by regulating the miR-216b/IGF2BP2 axis. Biomark Res 2022; 10:60. [PMID: 35971159 PMCID: PMC9380392 DOI: 10.1186/s40364-022-00408-x] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 05/06/2022] [Accepted: 08/02/2022] [Indexed: 11/10/2022] Open
Abstract
Introduction Esophageal squamous cell carcinoma (ESCC) represents a major malignancy with poor clinical outcomes. Long noncoding RNAs (lncRNAs) are known to regulate the development and progression of multiple cancers. However, how lncRNAs are involved in ESCC is currently undefined. Methods LIPH-4 levels in ESCC tissue specimens and cells were assessed by qRT-PCR. The biological function of LIPH-4 was examined in cell and animal studies, applying CCK-8, EdU, colony formation and flow cytometry assays as well as xenograft model experiments. The underlying mechanisms of action of LIPH-4 were explored through bioinformatics, luciferase reporter assay, RNA-immunoprecipitation assay and immunoblot. Results We identified a novel lncRNA, LIPH-4, which showed elevated amounts in ESCC tissues and positive correlations with increased tumor size and poor prognosis in ESCC patients. Functional studies showed that LIPH-4 promoted the growth, mediated cell cycle progression and inhibited apoptosis in ESCC cells in vitro, and promoted tumor growth in mice. In terms of mechanism, LIPH-4 could bind to miR-216b and act as a competing endogenous RNA (ceRNA) to induce the expression of miR-216’s target gene IGF2BP2. LIPH-4 played an oncogenic role in ESCC through the miR-216b/IGF2BP2 axis. Conclusions This study suggested that LIPH-4 functions as a novel oncogenic lncRNA by acting as a ceRNA for miR-216b to regulate IGF2BP2, indicating LIPH-4 likely constitutes a prognostic biomarker and therapeutic target in ESCC. Supplementary Information The online version contains supplementary material available at 10.1186/s40364-022-00408-x.
Collapse
Affiliation(s)
- Yuhang Xiao
- Hunan Clinical Medical Research Center of Accurate Diagnosis and Treatment for Esophageal Carcinoma, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.,Department of Pharmacy, Xiangya Hospital of Xiangya School of Medicine, Central South University, Changsha, China
| | - Jinming Tang
- Hunan Clinical Medical Research Center of Accurate Diagnosis and Treatment for Esophageal Carcinoma, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.,Thoracic Surgery Department 2, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
| | - Desong Yang
- Hunan Clinical Medical Research Center of Accurate Diagnosis and Treatment for Esophageal Carcinoma, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.,Thoracic Surgery Department 2, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
| | - Baihua Zhang
- Hunan Clinical Medical Research Center of Accurate Diagnosis and Treatment for Esophageal Carcinoma, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.,Thoracic Surgery Department 2, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
| | - Jie Wu
- Hunan Clinical Medical Research Center of Accurate Diagnosis and Treatment for Esophageal Carcinoma, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.,Thoracic Surgery Department 2, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
| | - Zhining Wu
- Hunan Clinical Medical Research Center of Accurate Diagnosis and Treatment for Esophageal Carcinoma, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.,Thoracic Surgery Department 2, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
| | - Qianjin Liao
- Hunan Key Laboratory of Cancer Metabolism, Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
| | - Hui Wang
- Key Laboratory of Translational Radiation Oncology, Department of Radiation Oncology, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
| | - Wenxiang Wang
- Hunan Clinical Medical Research Center of Accurate Diagnosis and Treatment for Esophageal Carcinoma, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.,Thoracic Surgery Department 2, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
| | - Min Su
- Hunan Clinical Medical Research Center of Accurate Diagnosis and Treatment for Esophageal Carcinoma, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China. .,Thoracic Surgery Department 2, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China. .,Hunan Key Laboratory of Cancer Metabolism, Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.
| |
Collapse
|
|
100
|
Deng W, Fan W, Li P, Yao J, Qi J, Chi H, Ji G, Zhao J. microRNA-497-mediated Smurf2/YY1/HIF2α axis in tumor growth and metastasis of esophageal squamous cell carcinoma. J Biochem Mol Toxicol 2022; 36:e23182. [PMID: 35938691 DOI: 10.1002/jbt.23182] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/19/2021] [Revised: 06/08/2022] [Accepted: 07/21/2022] [Indexed: 11/12/2022]
Abstract
Aberrant expression of microRNA-497 (miR-497) is associated with tumor progression, but the molecular mechanisms in tumorigenesis remain largely unknown. Here, we report that miR-497 expression is downregulated in esophageal squamous cell carcinoma (ESCC) clinical samples. Consistently, upregulation of miR-497 inhibits ESCC cell malignant properties and tumor growth in vivo. Importantly, we uncovered that miR-497 upregulation suppressed ESCC cell growth and tumor growth by inhibiting Smurf2. Mechanistically, we showed that Smurf2 was a target of miR-497, and mediated YY1 expression to elevate HIF2α expression, thereby enhancing the malignancy of ESCC cells. Together, our study uncovered the role of the miR-497-mediated Smurf2/YY1/HIF2α axis in tumor growth and metastasis, which might provide potential therapeutic targets for human ESCC.
Collapse
Affiliation(s)
- Weijun Deng
- Department of Thoracic Surgery, First Affiliated Hospital of Soochow University, Suzhou, China.,Department of Thoracic Surgery, Lianshui County People's Hospital, Huaian, China
| | - Wei Fan
- Department of Clinical Laboratory, Huai'an Tumor Hospital & Huai'an Hospital of Huai'an City, Huaian, China
| | - Peng Li
- Department of Radiotherapy, Huai'an Tumor Hospital & Huai'an Hospital of Huai'an City, Huaian, China
| | - Juan Yao
- Department of Radiotherapy, Huai'an Tumor Hospital & Huai'an Hospital of Huai'an City, Huaian, China
| | - Jinyou Qi
- Department of Clinical Laboratory, Huai'an Tumor Hospital & Huai'an Hospital of Huai'an City, Huaian, China
| | - Hao Chi
- Department of Clinical Laboratory, Huai'an Tumor Hospital & Huai'an Hospital of Huai'an City, Huaian, China
| | - Guoxian Ji
- Department of Radiotherapy, Huai'an Tumor Hospital & Huai'an Hospital of Huai'an City, Huaian, China
| | - Jun Zhao
- Department of Thoracic Surgery, First Affiliated Hospital of Soochow University, Suzhou, China
| |
Collapse
|