|
1
|
Hua H, Yang X, Meng D, Gan R, Chen N, He L, Wang D, Jiang W, Si D, Wang X, Zhang X, Wei X, Wang Y, Li B, Zhang H, Gao C. CTSG restraines the proliferation and metastasis of head and neck squamous cell carcinoma by blocking the JAK2/STAT3 pathway. Cell Signal 2025; 127:111562. [PMID: 39672353 DOI: 10.1016/j.cellsig.2024.111562] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/19/2024] [Revised: 11/24/2024] [Accepted: 12/09/2024] [Indexed: 12/15/2024]
Abstract
BACKGROUND Head and neck squamous cell carcinoma (HNSC) is recognized as the sixth most prevalent cancer globally, with around 900,000 new cases diagnosed each year. The management of HNSC poses significant challenges due to its rising incidence and suboptimal treatment outcomes in many patients. Thus, understanding the underlying molecular mechanisms that drive the onset and advancement of HNSC is crucial in order to steer the creation of novel treatment strategies. Previous researches have suggested that Cathepsin G (CTSG), a serine protease, may play a role in tumorigenesis, but its exact function in HNSC is still unknown. METHODS The TCGA and GTEx datasets were utilized to examine the expression and potential role of CTSG in pancancer. CTSG expression in HNSC tissues and normal tissues was analyzed using qRT-PCR, Western blot and immunohistochemistry techniques. The effects of altering CTSG expression on proliferation, migration, and apoptosis of HNSC cells were evaluated using various tests such as MTT assays, colony formation assays, wound-healing assays, transwell assays, flow cytometry, and xenograft tumor growth models. The functionality of CTSG on the JAK2/STAT3 pathway was validated using activators and inhibitors of this pathway after comfirming that CTSG could regulate this pathway. RESULTS In our study, we indicated that CTSG expression in HNSC tumor tissues was significantly lower than in adjacent normal tissues and CTSG gene level was positively correlated with patient prognosis. Additionally, we observed a decrease in tumor proliferation and migration, as well as an increase in apoptosis, following CTSG overexpression. Conversely, opposite effects were noted upon CTSG knockdown. Mechanistically, CTSG overexpression inhibited JAK2/STAT3 signaling, while CTSG knockdown activated it. This was confirmed by using IL-6 and JAK2 inhibitor. CONCLUSION CTSG impedes the proliferation and metastasis of HNSC in vivo and in vitro. CTSG is potential to act as a cancer suppressor in HNSC by focusing on the JAK2/STAT3 signaling pathway, indicating its possible use as a diagnostic marker and treatment target for HNSC.
Collapse
Affiliation(s)
- Hongting Hua
- Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China
| | - Xiaonan Yang
- Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China
| | - Dongdong Meng
- Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China
| | - Ruijia Gan
- Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China
| | - Nuo Chen
- Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China
| | - Lanqiaofeng He
- Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China
| | - Dong Wang
- Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China
| | - Wanjin Jiang
- Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Wannan Medical College, Wuhu 241000, China
| | - Dongyu Si
- Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China
| | - Xu Wang
- Department of General Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China
| | - Xiaomin Zhang
- Department of Biochemistry & Molecular Biology, Metabolic Disease Research Center, School of Basic Medicine, Anhui Medical University, Hefei 230032, Anhui, China
| | - Xiang Wei
- Department of Biochemistry & Molecular Biology, Metabolic Disease Research Center, School of Basic Medicine, Anhui Medical University, Hefei 230032, Anhui, China
| | - Yiming Wang
- Department of Biochemistry & Molecular Biology, Metabolic Disease Research Center, School of Basic Medicine, Anhui Medical University, Hefei 230032, Anhui, China
| | - Bao Li
- Synthetic Laboratory of School of Basic Medicine Sciences, Anhui Medical University, Hefei 230032, China
| | - Huabing Zhang
- Department of Biochemistry & Molecular Biology, Metabolic Disease Research Center, School of Basic Medicine, Anhui Medical University, Hefei 230032, Anhui, China.
| | - Chaobing Gao
- Department of Otorhinolaryngology Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, Anhui, China.
| |
Collapse
|
|
2
|
Wei Z, Hu X, Wu Y, Zhou L, Zhao M, Lin Q. Molecular Mechanisms Underlying Initiation and Activation of Autophagy. Biomolecules 2024; 14:1517. [PMID: 39766224 PMCID: PMC11673044 DOI: 10.3390/biom14121517] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/04/2024] [Revised: 11/15/2024] [Accepted: 11/26/2024] [Indexed: 01/11/2025] Open
Abstract
Autophagy is an important catabolic process to maintain cellular homeostasis and antagonize cellular stresses. The initiation and activation are two of the most important aspects of the autophagic process. This review focuses on mechanisms underlying autophagy initiation and activation and signaling pathways regulating the activation of autophagy found in recent years. These findings include autophagy initiation by liquid-liquid phase separation (LLPS), autophagy initiation in the endoplasmic reticulum (ER) and Golgi apparatus, and the signaling pathways mediated by the ULK1 complex, the mTOR complex, the AMPK complex, and the PI3KC3 complex. Through the review, we attempt to present current research progress in autophagy regulation and forward our understanding of the regulatory mechanisms and signaling pathways of autophagy initiation and activation.
Collapse
Affiliation(s)
| | | | | | | | | | - Qiong Lin
- School of Medicine, Jiangsu University, 301 Xuefu Road, Zhenjiang 212013, China; (Z.W.); (X.H.); (Y.W.); (L.Z.); (M.Z.)
| |
Collapse
|
|
3
|
Lee HW, Chen SJ, Tsai KJ, Hsu KS, Chen YF, Chang CH, Lin HH, Hsueh WY, Hsieh HP, Lee YF, Chiang HC, Chang JY. Targeting cathepsin S promotes activation of OLF1-BDNF/TrkB axis to enhance cognitive function. J Biomed Sci 2024; 31:46. [PMID: 38725007 PMCID: PMC11084077 DOI: 10.1186/s12929-024-01037-2] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/27/2023] [Accepted: 04/27/2024] [Indexed: 05/12/2024] Open
Abstract
BACKGROUND Cathepsin S (CTSS) is a cysteine protease that played diverse roles in immunity, tumor metastasis, aging and other pathological alterations. At the cellular level, increased CTSS levels have been associated with the secretion of pro-inflammatory cytokines and disrupted the homeostasis of Ca2+ flux. Once CTSS was suppressed, elevated levels of anti-inflammatory cytokines and changes of Ca2+ influx were observed. These findings have inspired us to explore the potential role of CTSS on cognitive functions. METHODS We conducted classic Y-maze and Barnes Maze tests to assess the spatial and working memory of Ctss-/- mice, Ctss+/+ mice and Ctss+/+ mice injected with the CTSS inhibitor (RJW-58). Ex vivo analyses including long-term potentiation (LTP), Golgi staining, immunofluorescence staining of sectioned whole brain tissues obtained from experimental animals were conducted. Furthermore, molecular studies were carried out using cultured HT-22 cell line and primary cortical neurons that treated with RJW-58 to comprehensively assess the gene and protein expressions. RESULTS Our findings reported that targeting cathepsin S (CTSS) yields improvements in cognitive function, enhancing both working and spatial memory in behavior models. Ex vivo studies showed elevated levels of long-term potentiation levels and increased synaptic complexity. Microarray analysis demonstrated that brain-derived neurotrophic factor (BDNF) was upregulated when CTSS was knocked down by using siRNA. Moreover, the pharmacological blockade of the CTSS enzymatic activity promoted BDNF expression in a dose- and time-dependent manner. Notably, the inhibition of CTSS was associated with increased neurogenesis in the murine dentate gyrus. These results suggested a promising role of CTSS modulation in cognitive enhancement and neurogenesis. CONCLUSION Our findings suggest a critical role of CTSS in the regulation of cognitive function by modulating the Ca2+ influx, leading to enhanced activation of the BDNF/TrkB axis. Our study may provide a novel strategy for improving cognitive function by targeting CTSS.
Collapse
Affiliation(s)
- Hao-Wei Lee
- Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Zhunan, Taiwan
- Taipei Cancer Center, TMU Research Center of Cancer Translational Medicine, Taipei Medical University Hospital, College of Medicine, Taipei Medical University, No. 252, Wuxing St., Xinyi Dist., Taipei, 110301, Taiwan (R.O.C.)
| | - Szu-Jung Chen
- Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Zhunan, Taiwan
| | - Kuen-Jer Tsai
- Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- Research Center of Clinical Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
| | - Kuei-Sen Hsu
- Institute of Basic Medical Science, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- Department of Pharmacology, College of Medicine, National Cheng Kung University, Tainan, Taiwan
| | - Yi-Fan Chen
- Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Zhunan, Taiwan
| | - Chih-Hua Chang
- Department of Pharmacology, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- Department of Biotechnology and Bioindustry Sciences, National Cheng Kung University, Tainan, Taiwan
| | - Hsiao-Han Lin
- Immunology Research Center, National Health Research Institutes, Zhunan, Taiwan
| | - Wen-Yun Hsueh
- Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Zhunan, Taiwan
| | - Hsing-Pang Hsieh
- Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Zhunan, Taiwan
| | - Yueh-Feng Lee
- Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Zhunan, Taiwan
| | - Huai-Chueh Chiang
- Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Zhunan, Taiwan
| | - Jang-Yang Chang
- Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Zhunan, Taiwan.
- Taipei Cancer Center, TMU Research Center of Cancer Translational Medicine, Taipei Medical University Hospital, College of Medicine, Taipei Medical University, No. 252, Wuxing St., Xinyi Dist., Taipei, 110301, Taiwan (R.O.C.).
| |
Collapse
|
|
4
|
Voronina MV, Frolova AS, Kolesova EP, Kuldyushev NA, Parodi A, Zamyatnin AA. The Intricate Balance between Life and Death: ROS, Cathepsins, and Their Interplay in Cell Death and Autophagy. Int J Mol Sci 2024; 25:4087. [PMID: 38612897 PMCID: PMC11012956 DOI: 10.3390/ijms25074087] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 02/06/2024] [Revised: 03/29/2024] [Accepted: 04/03/2024] [Indexed: 04/14/2024] Open
Abstract
Cellular survival hinges on a delicate balance between accumulating damages and repair mechanisms. In this intricate equilibrium, oxidants, currently considered physiological molecules, can compromise vital cellular components, ultimately triggering cell death. On the other hand, cells possess countermeasures, such as autophagy, which degrades and recycles damaged molecules and organelles, restoring homeostasis. Lysosomes and their enzymatic arsenal, including cathepsins, play critical roles in this balance, influencing the cell's fate toward either apoptosis and other mechanisms of regulated cell death or autophagy. However, the interplay between reactive oxygen species (ROS) and cathepsins in these life-or-death pathways transcends a simple cause-and-effect relationship. These elements directly and indirectly influence each other's activities, creating a complex web of interactions. This review delves into the inner workings of regulated cell death and autophagy, highlighting the pivotal role of ROS and cathepsins in these pathways and their intricate interplay.
Collapse
Affiliation(s)
- Maya V. Voronina
- Research Center for Translational Medicine, Sirius University of Science and Technology, 354340 Sochi, Russia; (M.V.V.); (A.S.F.); (E.P.K.); (N.A.K.); (A.P.)
| | - Anastasia S. Frolova
- Research Center for Translational Medicine, Sirius University of Science and Technology, 354340 Sochi, Russia; (M.V.V.); (A.S.F.); (E.P.K.); (N.A.K.); (A.P.)
- Institute of Translational Medicine and Biotechnology, Sechenov First Moscow State Medical University, 119991 Moscow, Russia
| | - Ekaterina P. Kolesova
- Research Center for Translational Medicine, Sirius University of Science and Technology, 354340 Sochi, Russia; (M.V.V.); (A.S.F.); (E.P.K.); (N.A.K.); (A.P.)
| | - Nikita A. Kuldyushev
- Research Center for Translational Medicine, Sirius University of Science and Technology, 354340 Sochi, Russia; (M.V.V.); (A.S.F.); (E.P.K.); (N.A.K.); (A.P.)
| | - Alessandro Parodi
- Research Center for Translational Medicine, Sirius University of Science and Technology, 354340 Sochi, Russia; (M.V.V.); (A.S.F.); (E.P.K.); (N.A.K.); (A.P.)
| | - Andrey A. Zamyatnin
- Faculty of Bioengineering and Bioinformatics, Lomonosov Moscow State University, 119234 Moscow, Russia
- Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119992 Moscow, Russia
- Department of Biological Chemistry, Sechenov First Moscow State Medical University, 119991 Moscow, Russia
| |
Collapse
|
|
5
|
Liu Y, Li Y, Jiang Y, Zheng X, Wang T, Li J, Zhang B, Zhu J, Wei X, Huang R, Zhang Y, Jin Q. Quercetin Promotes Apoptosis of Gastric Cancer Cells through the EGFR-ERK Signaling Pathway. J Food Biochem 2024; 2024:1-23. [DOI: 10.1155/2024/9945178] [Citation(s) in RCA: 1] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 01/02/2025]
Abstract
Previous studies have shown that various active components of licorice have anticancer effects. However, few studies have investigated the mechanism of action of licorice in gastric cancer. The effect of active compounds in licorice on the biological activity of gastric cancer cells was investigated in vitro (MKN-45 cells). Network pharmacology and molecular docking were used to predict the potential targets of licorice against gastric cancer and verify the binding stability of target proteins to compounds. In addition, the anticancer effect of licorice was assessed using a mouse model of gastric cancer. The licorice-active component (quercetin) effectively inhibited proliferation, caused cell cycle arrest, and promoted apoptosis in MKN-45 cells, accompanied by increased Cyt-C, decreased BCL-2, and decreased mitochondrial membrane potential and mitochondrial damage. Further research showed that quercetin targeted EGFR, blocked the ERK signaling pathway, and downregulated PTGS2. In the in vivo experiment, quercetin treatment resulted in reduced tumor volume, decreased Ki67 and BCL-2 expression in tumor tissue, increased caspase 3 and BAX levels, and induced mitochondrial damage.
Collapse
Affiliation(s)
- Yali Liu
- College of Veterinary Medicine, Gansu Agricultural University, Lanzhou, China
- The Second Hospital & Clinical Medical School, Lanzhou University, Lanzhou, China
| | - Yan Li
- The Second Hospital & Clinical Medical School, Lanzhou University, Lanzhou, China
| | - Yanjun Jiang
- Gansu Jiantou Technology Research and Development Co., Ltd., Lanzhou, China
| | - Xin Zheng
- The Second Hospital & Clinical Medical School, Lanzhou University, Lanzhou, China
| | | | - Jing Li
- The Second Hospital & Clinical Medical School, Lanzhou University, Lanzhou, China
| | - Biyun Zhang
- Tianshui Fourth People’s Hospital, Tianshui, China
| | - Jiarui Zhu
- The Second Hospital & Clinical Medical School, Lanzhou University, Lanzhou, China
| | - Xintong Wei
- The Second Clinical Medical College, Lanzhou University, Lanzhou, China
| | - Ruihua Huang
- The Second Clinical Medical College, Lanzhou University, Lanzhou, China
| | - Yong Zhang
- College of Veterinary Medicine, Gansu Agricultural University, Lanzhou, China
| | - Qiaoying Jin
- The Second Hospital & Clinical Medical School, Lanzhou University, Lanzhou, China
| |
Collapse
|
|
6
|
Govindasamy B, Muthu M, Gopal J, Chun S. A review on the impact of TRAIL on cancer signaling and targeting via phytochemicals for possible cancer therapy. Int J Biol Macromol 2023; 253:127162. [PMID: 37788732 DOI: 10.1016/j.ijbiomac.2023.127162] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/27/2022] [Revised: 09/11/2023] [Accepted: 09/28/2023] [Indexed: 10/05/2023]
Abstract
Anticancer therapies have been the continual pursuit of this age. Cancer has been ravaging all across the globe breathing not just threats but demonstrating them. Remedies for cancer have been frantically sought after. Few have worked out, yet till date, the available cancer therapies have not delivered a holistic solution. In a world where the search for therapies is levitating towards natural remedies, solutions based on phytochemicals are highly prospective attractions. A lot has been achieved with inputs from plant resources, providing numerous natural remedies. In the current review, we intensely survey the progress achieved in the treatment of cancer through phytochemicals-based programmed cell death of cancer cells. More specifically, we have further reviewed and discussed the role of phytochemicals in activating apoptosis via Tumor Necrosis Factor-Alpha-Related Apoptosis-Inducing Ligand (TRAIL), which is a cell protein that can attach to certain molecules in cancer cells, killing cancer cells. The objective of this review is to enlist the various phytochemicals that are available for specifically contributing towards triggering the TRAIL cell protein-mediated cancer therapy and to point out the research gaps that require future research motivation. This is the first review of this kind in this research direction.
Collapse
Affiliation(s)
- Balasubramani Govindasamy
- Department of Product Development, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences (SIMATS), Thandalam, Chennai 602105, India
| | - Manikandan Muthu
- Department of Research and Innovation, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences (SIMATS), Thandalam, Chennai 602105, India
| | - Judy Gopal
- Department of Research and Innovation, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences (SIMATS), Thandalam, Chennai 602105, India
| | - Sechul Chun
- Department of Bioresources and Food Science, Institute of Natural Science and Agriculture, Konkuk University, 1 Hwayang-dong, Gwangjin-gu, Seoul 05029, Republic of Korea.
| |
Collapse
|
|
7
|
Liu H, Peng J, Huang L, Ruan D, Li Y, Yuan F, Tu Z, Huang K, Zhu X. The role of lysosomal peptidases in glioma immune escape: underlying mechanisms and therapeutic strategies. Front Immunol 2023; 14:1154146. [PMID: 37398678 PMCID: PMC10311646 DOI: 10.3389/fimmu.2023.1154146] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/30/2023] [Accepted: 06/02/2023] [Indexed: 07/04/2023] Open
Abstract
Glioblastoma is the most common primary malignant tumor of the central nervous system, which has the characteristics of strong invasion, frequent recurrence, and rapid progression. These characteristics are inseparable from the evasion of glioma cells from immune killing, which makes immune escape a great obstacle to the treatment of glioma, and studies have confirmed that glioma patients with immune escape tend to have poor prognosis. The lysosomal peptidase lysosome family plays an important role in the immune escape process of glioma, which mainly includes aspartic acid cathepsin, serine cathepsin, asparagine endopeptidases, and cysteine cathepsins. Among them, the cysteine cathepsin family plays a prominent role in the immune escape of glioma. Numerous studies have confirmed that glioma immune escape mediated by lysosomal peptidases has something to do with autophagy, cell signaling pathways, immune cells, cytokines, and other mechanisms, especially lysosome organization. The relationship between protease and autophagy is more complicated, and the current research is neither complete nor in-depth. Therefore, this article reviews how lysosomal peptidases mediate the immune escape of glioma through the above mechanisms and explores the possibility of lysosomal peptidases as a target of glioma immunotherapy.
Collapse
Affiliation(s)
- Hao Liu
- Department of Neurosurgery, The Second Affifiliated Hospital of Nanchang University, Nanchang, China
- The Second Clinical Medical College of Nanchang University, Nanchang, China
| | - Jie Peng
- Department of Neurosurgery, The Second Affifiliated Hospital of Nanchang University, Nanchang, China
- The Second Clinical Medical College of Nanchang University, Nanchang, China
| | - Linzhen Huang
- The Second Clinical Medical College of Nanchang University, Nanchang, China
| | - Dong Ruan
- The Second Clinical Medical College of Nanchang University, Nanchang, China
| | - Yuguang Li
- The Second Clinical Medical College of Nanchang University, Nanchang, China
| | - Fan Yuan
- The Second Clinical Medical College of Nanchang University, Nanchang, China
| | - Zewei Tu
- Department of Neurosurgery, The Second Affifiliated Hospital of Nanchang University, Nanchang, China
- Jiangxi Key Laboratory of Neurological Tumors and Cerebrovascular Diseases, Nanchang, China
- Institute of Neuroscience, Nanchang University, Nanchang, China
- Jiangxi Health Commission (JXHC) Key Laboratory of Neurological Medicine, Nanchang, China
| | - Kai Huang
- Department of Neurosurgery, The Second Affifiliated Hospital of Nanchang University, Nanchang, China
- Jiangxi Key Laboratory of Neurological Tumors and Cerebrovascular Diseases, Nanchang, China
- Institute of Neuroscience, Nanchang University, Nanchang, China
- Jiangxi Health Commission (JXHC) Key Laboratory of Neurological Medicine, Nanchang, China
| | - Xingen Zhu
- Department of Neurosurgery, The Second Affifiliated Hospital of Nanchang University, Nanchang, China
- Jiangxi Key Laboratory of Neurological Tumors and Cerebrovascular Diseases, Nanchang, China
- Institute of Neuroscience, Nanchang University, Nanchang, China
- Jiangxi Health Commission (JXHC) Key Laboratory of Neurological Medicine, Nanchang, China
| |
Collapse
|
|
8
|
Wang XY, Beeraka NM, Xue NN, Yu HM, Yang Y, Liu MX, Nikolenko VN, Liu JQ, Zhao D. Identification of a three-gene prognostic signature for radioresistant esophageal squamous cell carcinoma. World J Clin Oncol 2023; 14:13-26. [PMID: 36699628 PMCID: PMC9850665 DOI: 10.5306/wjco.v14.i1.13] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 07/25/2022] [Revised: 10/25/2022] [Accepted: 12/06/2022] [Indexed: 01/10/2023] Open
Abstract
BACKGROUND Esophageal squamous cell carcinoma (ESCC) is causing a high mortality rate due to the lack of efficient early prognosis markers and suitable therapeutic regimens. The prognostic role of genes responsible for the acquisition of radioresistance in ESCC has not been fully elucidated.
AIM To establish a prognostic model by studying gene expression patterns pertinent to radioresistance in ESCC patients.
METHODS Datasets were obtained from the Gene Expression Omnibus and The Cancer Genome Atlas databases. The edgeR, a Bioconductor package, was used to analyze mRNA expression between different groups. We screened genes specifically responsible for radioresistance to estimate overall survival. Pearson correlation analysis was performed to confirm whether the expression of those genes correlated with each other. Genes contributing to radioresistance and overall survival were assessed by the multivariate Cox regression model through the calculation of βi and risk score using the following formula: 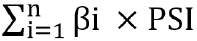 . .
RESULTS We identified three prognostic mRNAs (cathepsin S [CTSS], cluster of differentiation 180 [CD180], and SLP adapter and CSK-interacting membrane protein [SCIMP]) indicative of radioresistance. The expression of the three identified mRNAs was related to each other (r > 0.70 and P < 0.05). As to 1-year and 3-year overall survival prediction, the area under the time-dependent receiver operating characteristic curve of the signature consisting of the three mRNAs was 0.716 and 0.841, respectively. When stratifying patients based on the risk score derived from the signature, the high-risk group exhibited a higher death risk and shorter survival time than the low-risk group (P < 0.0001). Overall survival of the low-risk patients was significantly better than that of the high-risk patients (P = 0.018).
CONCLUSION We have developed a novel three-gene prognostic signature consisting of CTSS, CD180, and SCIMO for ESCC, which may facilitate the prediction of early prognosis of this malignancy.
Collapse
Affiliation(s)
- Xiao-Yan Wang
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Narasimha M Beeraka
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- Department of Pharmaceutical Chemistry, JSS College of Pharmacy, Mysuru 570015, India
| | - Nan-Nan Xue
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Hui-Ming Yu
- Department of Radiation Oncology, Peking University Cancer Hospital & Institute, Beijing 065005, China
| | - Ya Yang
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Mao-Xing Liu
- Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Gastrointestinal Surgery IV, Peking University Cancer Hospital & Institute, Beijing, China
| | - Vladimir N Nikolenko
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- M.V. Lomonosov Moscow State University, Moscow 119991, Russia
| | - Jun-Qi Liu
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Di Zhao
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| |
Collapse
|
|
9
|
Smyth P, Sasiwachirangkul J, Williams R, Scott CJ. Cathepsin S (CTSS) activity in health and disease - A treasure trove of untapped clinical potential. Mol Aspects Med 2022; 88:101106. [PMID: 35868042 DOI: 10.1016/j.mam.2022.101106] [Citation(s) in RCA: 55] [Impact Index Per Article: 18.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 02/28/2022] [Revised: 06/24/2022] [Accepted: 07/11/2022] [Indexed: 12/14/2022]
Abstract
Amongst the lysosomal cysteine cathepsin family of proteases, cathepsin S (CTSS) holds particular interest due to distinctive properties including a normal restricted expression profile, inducible upregulation and activity at a broad pH range. Consequently, while CTSS is well-established as a member of the proteolytic cocktail within the lysosome, degrading unwanted and damaged proteins, it has increasingly been shown to mediate a number of distinct, more selective roles including antigen processing and antigen presentation, and cleavage of substrates both intra and extracellularly. Increasingly, aberrant CTSS expression has been demonstrated in a variety of conditions and disease states, marking it out as both a biomarker and potential therapeutic target. This review seeks to contextualise CTSS within the cysteine cathepsin family before providing an overview of the broad range of pathologies in which roles for CTSS have been identified. Additionally, current clinical progress towards specific inhibitors is detailed, updating the position of the field in exploiting this most unique of proteases.
Collapse
Affiliation(s)
- Peter Smyth
- The Patrick G Johnston Centre for Cancer Research, Queen's University, 97 Lisburn Road, Belfast, BT9 7AE, UK
| | - Jutharat Sasiwachirangkul
- The Patrick G Johnston Centre for Cancer Research, Queen's University, 97 Lisburn Road, Belfast, BT9 7AE, UK
| | - Rich Williams
- The Patrick G Johnston Centre for Cancer Research, Queen's University, 97 Lisburn Road, Belfast, BT9 7AE, UK
| | - Christopher J Scott
- The Patrick G Johnston Centre for Cancer Research, Queen's University, 97 Lisburn Road, Belfast, BT9 7AE, UK.
| |
Collapse
|
|
10
|
Ghanadi K, Ashorzadeh S, Aliyepoor A, Anbari K. Evaluation of serum levels of cathepsin S among colorectal cancer patients. Ann Med Surg (Lond) 2022; 78:103831. [PMID: 35734720 PMCID: PMC9206904 DOI: 10.1016/j.amsu.2022.103831] [Citation(s) in RCA: 2] [Impact Index Per Article: 0.7] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/12/2022] [Revised: 05/16/2022] [Accepted: 05/17/2022] [Indexed: 11/24/2022] Open
Abstract
Objective Colorectal cancer is the third most common cancer worldwide. Cathepsins are protease that are known to be involved in cancer progression and metastasis. The aim of this study is to evaluate the levels of serum cathepsin S in patients and control subjects and its effects on the prognosis of the cancer. Methods In this case-control study, colorectal cancer patients referred to our gastroenterology clinic were included. The control group consisted of healthy individuals. Cathepsin S levels were analyzed in these patients and the check list consisting of demographic data, cancer stage, colonoscopy findings, CEA marker and cathepsin S levels were recorded. Results Of 80 patients and healthy controls included in the study, age, gender and BMI were not significantly different among the two groups, p = 0.265, p = 0.752 and p = 0.2, respectively. Cathepsin S levels were significantly greater in-patient group p < 0.001 and was significantly correlated with the stage of the tumor. CEA marker was also linear related with the increased levels of cathepsin S, p < 0.001. Conclusion Our study concluded that cathepsin S is elevated in the cancer patients and can be a significant marker for the prognosis of colorectal cancer.
Collapse
Affiliation(s)
- Koroush Ghanadi
- Hepatitis Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran
| | - Saber Ashorzadeh
- Student Research Committee, Lorestan University of Medical Sciences, Khorramabad, Iran
| | - Asghar Aliyepoor
- Department of Pathology, Faculty of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran
| | - Khatereh Anbari
- Social Determinants of Health Research Center, Faculty of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran
| |
Collapse
|
|
11
|
Kos J, Mitrović A, Perišić Nanut M, Pišlar A. Lysosomal peptidases – Intriguing roles in cancer progression and neurodegeneration. FEBS Open Bio 2022; 12:708-738. [PMID: 35067006 PMCID: PMC8972049 DOI: 10.1002/2211-5463.13372] [Citation(s) in RCA: 2] [Impact Index Per Article: 0.7] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/08/2021] [Revised: 01/04/2022] [Accepted: 01/20/2022] [Indexed: 11/16/2022] Open
Abstract
Lysosomal peptidases are hydrolytic enzymes capable of digesting waste proteins that are targeted to lysosomes via endocytosis and autophagy. Besides intracellular protein catabolism, they play more specific roles in several other cellular processes and pathologies, either within lysosomes, upon secretion into the cell cytoplasm or extracellular space, or bound to the plasma membrane. In cancer, lysosomal peptidases are generally associated with disease progression, as they participate in crucial processes leading to changes in cell morphology, signaling, migration, and invasion, and finally metastasis. However, they can also enhance the mechanisms resulting in cancer regression, such as apoptosis of tumor cells or antitumor immune responses. Lysosomal peptidases have also been identified as hallmarks of aging and neurodegeneration, playing roles in oxidative stress, mitochondrial dysfunction, abnormal intercellular communication, dysregulated trafficking, and the deposition of protein aggregates in neuronal cells. Furthermore, deficiencies in lysosomal peptidases may result in other pathological states, such as lysosomal storage disease. The aim of this review was to highlight the role of lysosomal peptidases in particular pathological processes of cancer and neurodegeneration and to address the potential of lysosomal peptidases in diagnosing and treating patients.
Collapse
Affiliation(s)
- Janko Kos
- University of Ljubljana Faculty of Pharmacy Aškerčeva 7 1000 Ljubljana Slovenia
- Jožef Stefan Institute Department of Biotechnology Jamova 39 1000 Ljubljana Slovenia
| | - Ana Mitrović
- Jožef Stefan Institute Department of Biotechnology Jamova 39 1000 Ljubljana Slovenia
| | - Milica Perišić Nanut
- Jožef Stefan Institute Department of Biotechnology Jamova 39 1000 Ljubljana Slovenia
| | - Anja Pišlar
- University of Ljubljana Faculty of Pharmacy Aškerčeva 7 1000 Ljubljana Slovenia
| |
Collapse
|
|
12
|
Gureeva TA, Timoshenko OS, Kugaevskaya EV, Solovyova NI. [Cysteine cathepsins: structure, physiological functions and their role in carcinogenesis]. BIOMEDITSINSKAIA KHIMIIA 2021; 67:453-464. [PMID: 34964439 DOI: 10.18097/pbmc20216706453] [Citation(s) in RCA: 3] [Impact Index Per Article: 0.8] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Subscribe] [Scholar Register] [Indexed: 01/13/2023]
Abstract
Cysteine cathepsins (Cts) also known as thiol proteinases belong to the superfamily of cysteine proteinases (EC 3.4.22). Cts are known as lysosomal proteases responsible for the intracellular proteins degradation. All Cts are synthesized as zymogens, activation of which occurs autocatalytically. Their activity is regulated by endogenous inhibitors. Cts can be secreted into the extracellular environment, which is of particular importance in tumor progression. Extracellular Cts not only hydrolyze extracellular matrix (ECM) proteins, but also contribute to ECM remodeling, processing and/or release of cell adhesion molecules, growth factors, cytokines and chemokines. In cancer, the expression and activity of Cts sharply increase both in cell lysosomes and in the intercellular space, which correlates with neoplastic transformation, invasion, metastasis and leads to further tumor progression. It has been shown that Cts expression depends on the cells type, therefore, their role in the tumor development differs depending on their cellular origin. The mechanism of Cts action in cancer is not limited only by their proteolytic action. The Cts influence on signal transduction pathways associated with cancer development, including the pathway involving growth factors, which is mediated through receptors tyrosine kinases (RTK) and various signaling mitogen-activated protein kinases (MAPK), has been proven. In addition, Cts are able to promote the epithelial-mesenchymal transition (EMT) by activating signal transduction pathways such as Wnt, Notch, and the pathway involving TGF-β. So, Ctc perform specific both destructive and regulatory functions, carrying out proteolysis, both inside and outside the cell.
Collapse
Affiliation(s)
- T A Gureeva
- Institute of Biomedical Chemistry, Moscow, Russia
| | | | | | | |
Collapse
|
|
13
|
Cao W, Gao J, Zhang Y, Li A, Yu P, Cao N, Liang J, Tang X. Autophagy up-regulated by MEK/ERK promotes the repair of DNA damage caused by aflatoxin B1. Toxicol Mech Methods 2021; 32:87-96. [PMID: 34396909 DOI: 10.1080/15376516.2021.1968985] [Citation(s) in RCA: 6] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 10/20/2022]
Abstract
Aflatoxin B1 (AFB1), a kind of mycotoxin, exerts its cytotoxicity by increasing the oxidative damage of target organs, especially the liver. In vivo and in vitro experiments were carried out to elucidate the toxic mechanism of AFB1. The results of MTT, cloning-formation, flow cytometry, immunocytochemistry, Reverse transcription PCR (RT-PCR) and western blot showed that AFB1 activated NOX2 gp91 phox, inhibited proliferation and migration, and blocked cell cycle at G0/G1 period of HHL-5 cells. Autophagy promoted the repair of NOX2-dependent DNA damage. NOX2/gp91 phox mainly activates MEK/ERK pathway and then up-regulates autophagy. In vivo experiments have shown that AFB1 (0.75 mg/kg daily orally, 4 weeks) had no significant changes in the size and shape of the liver in mice. However, these treatments lead to structural abnormalities of hepatocytes and DNA damage. In summary, AFB1 caused intracellular oxidative stress and DNA damage, NOX2/gp91-phox activates the MEK/ERK pathway, and upregulated autophagy to promote the repair of DNA damage. We concluded that by increasing the level of autophagy, the ability of anti-AFB1 toxicity of liver can be increased.
Collapse
Affiliation(s)
- Weiya Cao
- Medical School, Anhui University of Science and Technology, Huainan, China.,Institute of Environment-friendly Materials and Occupational Health, Anhui University of Science and Technology, Wuhu, China
| | - Jiafeng Gao
- Medical School, Anhui University of Science and Technology, Huainan, China.,Institute of Environment-friendly Materials and Occupational Health, Anhui University of Science and Technology, Wuhu, China
| | - Yinci Zhang
- Medical School, Anhui University of Science and Technology, Huainan, China.,Institute of Environment-friendly Materials and Occupational Health, Anhui University of Science and Technology, Wuhu, China
| | - Amin Li
- Medical School, Anhui University of Science and Technology, Huainan, China.,Institute of Environment-friendly Materials and Occupational Health, Anhui University of Science and Technology, Wuhu, China
| | - Pan Yu
- Medical School, Anhui University of Science and Technology, Huainan, China.,Institute of Environment-friendly Materials and Occupational Health, Anhui University of Science and Technology, Wuhu, China
| | - Niandie Cao
- Medical School, Anhui University of Science and Technology, Huainan, China.,Institute of Environment-friendly Materials and Occupational Health, Anhui University of Science and Technology, Wuhu, China
| | - Jiaojiao Liang
- Medical School, Anhui University of Science and Technology, Huainan, China.,Institute of Environment-friendly Materials and Occupational Health, Anhui University of Science and Technology, Wuhu, China
| | - Xiaolong Tang
- Medical School, Anhui University of Science and Technology, Huainan, China.,Institute of Environment-friendly Materials and Occupational Health, Anhui University of Science and Technology, Wuhu, China
| |
Collapse
|
|
14
|
Cheng SM, Shieh MC, Lin TY, Cheung CHA. The "Dark Side" of autophagy on the maintenance of genome stability: Does it really exist during excessive activation? J Cell Physiol 2021; 237:178-188. [PMID: 34406646 DOI: 10.1002/jcp.30555] [Citation(s) in RCA: 3] [Impact Index Per Article: 0.8] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 04/13/2021] [Revised: 07/13/2021] [Accepted: 08/06/2021] [Indexed: 01/18/2023]
Abstract
Dysregulation of DNA damage response/repair and genomic instability promote tumorigenesis and the development of various neurological diseases. Autophagy is a dynamic catabolic process used for removing unnecessary or dysfunctional proteins and organelles in cells. Despite the consensus in the field that upregulation of autophagy promotes the initiation of the DNA damage response and assists the process of homologous recombination upon genotoxic stress, a few studies showed that upregulation of autophagy (or excessive autophagy), under certain circumstances, triggers caspase/apoptosis-independent DNA damage and promotes genomic instability in cells. As the cytoprotective and the DNA repairing roles of autophagy have been discussed extensively in different reviews, here, we mainly focus on describing the latest studies which reported the "opposite" roles of autophagy (or excessive autophagy). We will discuss whether the "dark side" (i.e., the opposite/unconventional effect) of autophagy on the maintenance of DNA integrity and genomic stability really does exist in cells and if it does, will it be one of the yet-to-be-identified causes of cancer, in this review.
Collapse
Affiliation(s)
- Siao Muk Cheng
- National Institute of Cancer Research, National Health Research Institutes (NHRI), Tainan, Taiwan
| | - Min-Chieh Shieh
- Division of General Surgery, Department of Surgery, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan
| | - Tzu-Yu Lin
- Institute of Basic Medical Sciences, National Cheng Kung University, Tainan, Taiwan
| | - Chun Hei Antonio Cheung
- Institute of Basic Medical Sciences, National Cheng Kung University, Tainan, Taiwan
- Department of Pharmacology, National Cheng Kung University, Tainan, Taiwan
| |
Collapse
|
|
15
|
Rahman MA, Hannan MA, Dash R, Rahman MDH, Islam R, Uddin MJ, Sohag AAM, Rahman MH, Rhim H. Phytochemicals as a Complement to Cancer Chemotherapy: Pharmacological Modulation of the Autophagy-Apoptosis Pathway. Front Pharmacol 2021; 12:639628. [PMID: 34025409 PMCID: PMC8138161 DOI: 10.3389/fphar.2021.639628] [Citation(s) in RCA: 71] [Impact Index Per Article: 17.8] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/09/2020] [Accepted: 03/18/2021] [Indexed: 12/11/2022] Open
Abstract
Bioactive plant derived compounds are important for a wide range of therapeutic applications, and some display promising anticancer properties. Further evidence suggests that phytochemicals modulate autophagy and apoptosis, the two crucial cellular pathways involved in the underlying pathobiology of cancer development and regulation. Pharmacological targeting of autophagy and apoptosis signaling using phytochemicals therefore offers a promising strategy that is complementary to conventional cancer chemotherapy. In this review, we sought to highlight the molecular basis of the autophagic-apoptotic pathway to understand its implication in the pathobiology of cancer, and explore this fundamental cellular process as a druggable anticancer target. We also aimed to present recent advances and address the limitations faced in the therapeutic development of phytochemical-based anticancer drugs.
Collapse
Affiliation(s)
- Md. Ataur Rahman
- Center for Neuroscience, Korea Institute of Science and Technology (KIST), Seoul, South Korea
- Global Biotechnology & Biomedical Research Network (GBBRN), Department of Biotechnology and Genetic Engineering, Faculty of Biological Sciences, Islamic University, Kushtia, Bangladesh
| | - Md. Abdul Hannan
- Department of Anatomy, Dongguk University College of Medicine, Gyeongju, South Korea
- Department of Biochemistry and Molecular Biology, Bangladesh Agricultural University, Mymensingh, Bangladesh
| | - Raju Dash
- Department of Anatomy, Dongguk University College of Medicine, Gyeongju, South Korea
| | - MD. Hasanur Rahman
- Department of Biotechnology and Genetic Engineering, Bangabandhu Sheikh Mujibur Rahman Science and Technology University, Gopalganj, Bangladesh
- Graduate School of Pharmaceutical Sciences, College of Pharmacy, Ewha Womans University, Seoul, South Korea
| | - Rokibul Islam
- Department of Biotechnology and Genetic Engineering, Faculty of Biological Sciences, Islamic University, Kushtia, Bangladesh
- Department of Biochemistry, College of Medicine, Hallym University, Chuncheon-si, South Korea
| | - Md Jamal Uddin
- ABEx Bio-Research Center, Dhaka, Bangladesh
- Graduate School of Pharmaceutical Sciences, College of Pharmacy, Ewha Womans University, Seoul, South Korea
| | - Abdullah Al Mamun Sohag
- Department of Biochemistry and Molecular Biology, Bangladesh Agricultural University, Mymensingh, Bangladesh
| | - Md. Habibur Rahman
- Department of Global Medical Science, Wonju College of Medicine, Yonsei University, Seoul, South Korea
| | - Hyewhon Rhim
- Center for Neuroscience, Korea Institute of Science and Technology (KIST), Seoul, South Korea
- Division of Bio-Medical Science and Technology, KIST School, Korea University of Science and Technology (UST), Seoul, South Korea
| |
Collapse
|
|
16
|
Pires D, Valente S, Calado M, Mandal M, Azevedo-Pereira JM, Anes E. Repurposing Saquinavir for Host-Directed Therapy to Control Mycobacterium Tuberculosis Infection. Front Immunol 2021; 12:647728. [PMID: 33841429 PMCID: PMC8032898 DOI: 10.3389/fimmu.2021.647728] [Citation(s) in RCA: 19] [Impact Index Per Article: 4.8] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/30/2020] [Accepted: 03/08/2021] [Indexed: 11/13/2022] Open
Abstract
Despite the available antibiotics, tuberculosis (TB) has made its return since the 90’s of the last century as a global threat mostly due to co-infection with HIV, to the emergence of drug resistant strains and the lack of an effective vaccine. Host-directed strategies could be exploited to improve treatment efficacy, contain drug-resistant strains, improve immune responses and reduce disease severity. Macrophages in the lungs are often found infected with Mycobacterium tuberculosis (Mtb) and/or with HIV. The long-term survival of lung macrophages infected with Mtb or with HIV, together with their ability to produce viral particles, especially during TB, makes these niches major contributors to the pathogenicity of the infection. Among the available drugs to control HIV infection, protease inhibitors (PIs), acting at post-integrational stages of virus replication cycle, are the only drugs able to interfere with virus production and release from macrophages during chronic infection. For Mtb we recently found that the pathogen induces a general down-regulation of lysosomal proteases, helping bacteria to establish an intracellular niche in macrophages. Here we found that the PI saquinavir, contrary to ritonavir, is able to induce an increase of endolysosomal proteases activity especially of cathepsin S in Mtb infected macrophages and during co-infection with HIV. Our results indicate that saquinavir treatment of infected macrophages led not only to a significant intracellular killing of Mtb but also: (i) to an improved expression of the HLA class II antigen presentation machinery at the cell surface; (ii) to increased T-lymphocyte priming and proliferation; and (iii) to increased secretion of IFN-γ. All together the results indicate saquinavir as a potential host directed therapy for tuberculosis.
Collapse
Affiliation(s)
- David Pires
- Host-Pathogen Interactions Unit, Research Institute for Medicines, iMed-ULisboa, Faculty of Pharmacy, Universidade de Lisboa, Lisboa, Portugal
| | - Sofia Valente
- Host-Pathogen Interactions Unit, Research Institute for Medicines, iMed-ULisboa, Faculty of Pharmacy, Universidade de Lisboa, Lisboa, Portugal
| | - Marta Calado
- Host-Pathogen Interactions Unit, Research Institute for Medicines, iMed-ULisboa, Faculty of Pharmacy, Universidade de Lisboa, Lisboa, Portugal
| | - Manoj Mandal
- Host-Pathogen Interactions Unit, Research Institute for Medicines, iMed-ULisboa, Faculty of Pharmacy, Universidade de Lisboa, Lisboa, Portugal
| | - José Miguel Azevedo-Pereira
- Host-Pathogen Interactions Unit, Research Institute for Medicines, iMed-ULisboa, Faculty of Pharmacy, Universidade de Lisboa, Lisboa, Portugal
| | - Elsa Anes
- Host-Pathogen Interactions Unit, Research Institute for Medicines, iMed-ULisboa, Faculty of Pharmacy, Universidade de Lisboa, Lisboa, Portugal
| |
Collapse
|
|
17
|
Dang YF, Yang SH, Jiang XN, Gong FL, Yang XX, Cheng YN, Guo XL. Combination treatment strategies with a focus on rosiglitazone and adriamycin for insulin resistant liver cancer. J Drug Target 2021; 29:336-348. [PMID: 33115283 DOI: 10.1080/1061186x.2020.1844216] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 10/23/2022]
Abstract
Insulin resistance promotes the occurrence of liver cancer and decreases its chemosensitivity. Rosiglitazone (ROSI), a thiazolidinedione insulin sensitiser, could be used for diabetes with insulin resistance and has been reported to show anticancer effects on human malignant cells. In this paper, we investigated the combination of ROSI and chemotherapeutics on the growth and metastasis of insulin-resistant hepatoma. In vitro assay, ROSI significantly enhanced the inhibitory effects of adriamycin (ADR) on the proliferation, autophagy and migration of insulin-resistant hepatoma HepG2/IR cells via downregulation of EGFR/ERK and AKT/mTOR signalling pathway. In addition, ROSI promoted the apoptosis of HepG2/IR cells induced by ADR. In vivo assay, high fat and glucose diet and streptozotocin (STZ) induced insulin resistance in mice by increasing the body weight, fasting blood glucose (FBG) level, oral glucose tolerance, fasting insulin level and insulin resistance index. Both the growth of mouse liver cancer hepatoma H22 cells and serum FBG level in insulin resistant mice were significantly inhibited by combination of ROSI and ADR. Thus, ROSI and ADR in combination showed a stronger anti-tumour effect in insulin resistant hepatoma cells accompanying with glucose reduction and might represent an effective therapeutic strategy for liver cancer accompanied with insulin resistant diabetes.
Collapse
Affiliation(s)
- Yi-Fan Dang
- Department of Pharmacology, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, PR China
| | - Shao-Hui Yang
- Shandong Wendeng Osteopathic Hospital, Wendeng, PR China
| | - Xiao-Ning Jiang
- Department of Pharmacology, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, PR China
| | - Fu-Lian Gong
- Department of Pharmacology, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, PR China
| | - Xiao-Xia Yang
- Department of Pharmacology, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, PR China
| | - Yan-Na Cheng
- Department of Pharmacology, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, PR China
| | - Xiu-Li Guo
- Department of Pharmacology, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, PR China
| |
Collapse
|
|
18
|
Fei M, Zhang L, Wang H, Zhu Y, Niu W, Tang T, Han Y. Inhibition of Cathepsin S Induces Mitochondrial Apoptosis in Glioblastoma Cell Lines Through Mitochondrial Stress and Autophagosome Accumulation. Front Oncol 2021; 10:516746. [PMID: 33425712 PMCID: PMC7787074 DOI: 10.3389/fonc.2020.516746] [Citation(s) in RCA: 16] [Impact Index Per Article: 4.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/02/2019] [Accepted: 09/30/2020] [Indexed: 12/27/2022] Open
Abstract
Cathepsin S (CTSS), a lysosomal cysteine protease, is overexpressed in various cancers, including glioblastoma (GB). A high level of CTSS is associated with tumor progression and poor outcome in GB. However, the underlying mechanisms of its role in the biological characteristics of G5B remain to be elucidated. Here, we uncovered a potential role of CTSS in the lysosomes and mitochondria of GB cells (GBCs). Downregulation of CTSS in GBCs could increase the expression of autophagy-related proteins; however, there was no significant change in p62, suggesting autophagy blockade. Moreover, inhibition of CTSS increased the expression of mitochondrial calcium uniporter (MCU) and enhanced mitochondrial Ca2+ uptake ability, causing mitochondrial Ca2+ overload, the generation of copious reactive oxygen species (ROS) and eventual mitochondrial apoptosis. Additionally, elevated damage to mitochondria exacerbated the burden of autophagy. Finally, we found that silence of MCU could alleviate the inhibition of CTSS-induced autophagosome accumulation and mitochondrial stress. Collectively, these results demonstrate that CTSS plays an important role in the process of autophagic flux and mitochondrial functions in GBCs.
Collapse
Affiliation(s)
- Maoxing Fei
- Department of Neurosurgery, Jinling Hospital, Nanjing Medical University, Nanjing, China
| | - Li Zhang
- Department of Neurosurgery, Jinling Hospital, Medical School of Nanjing University, Nanjing, China
| | - Handong Wang
- Department of Neurosurgery, Jinling Hospital, Nanjing Medical University, Nanjing, China
| | - Yihao Zhu
- Department of Neurosurgery, Jinling Hospital, Medical School of Nanjing University, Nanjing, China
| | - Wenhao Niu
- Department of Neurosurgery, Jinling Hospital, School of Medicine, Southeast University, Nanjing, China
| | - Ting Tang
- Department of Neurosurgery, Jinling Hospital, Medical School of Nanjing University, Nanjing, China
| | - Yanling Han
- Department of Neurosurgery, Jinling Hospital, Nanjing, China
| |
Collapse
|
|
19
|
Kan LLY, Liu D, Chan BCL, Tsang MSM, Hou T, Leung PC, Lam CWK, Wong CK. The flavonoids of Sophora flavescens exerts anti-inflammatory activity via promoting autophagy of Bacillus Calmette-Guérin-stimulated macrophages. J Leukoc Biol 2020; 108:1615-1629. [PMID: 32794339 DOI: 10.1002/jlb.3ma0720-682rr] [Citation(s) in RCA: 8] [Impact Index Per Article: 1.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/30/2020] [Revised: 07/24/2020] [Accepted: 08/04/2020] [Indexed: 11/06/2022] Open
Abstract
Tuberculosis (TB), a highly infectious air-borne disease, has remained a global health problem. Conventional treatment and preventions such as antibiotics and Bacilli Calmette-Guerin (BCG) vaccine can be unreliable. In view of the increasing prevalence of anti-TB drug resistance, adjunctive therapy may be necessary to shorten the recovery time. We have previously shown that flavonoids in the medicinal herb Sophora flavescens exhibit anti-inflammatory and bactericidal activities. The aim of this study was to investigate the molecular and cellular characteristics of flavonoids of S. flavescens (FSF) in BCG-stimulated macrophages for assessing their roles in anti-inflammation and autophagy. Mouse alveolar macrophage (MH-S) cell line and primary mouse peritoneal macrophages were stimulated in vitro with heat-inactivated BCG and treated with FSF, with or without autophagy inhibitor Bafilomycin A1 (BafA1). Gene expression was analyzed using quantitative PCR, and cytokine/chemokine release was analyzed by Milliplex assay and ELISA. Autophagy-related proteins were quantified by Western blot and flow cytometry, and autophagolysosomes were detected using fluorescence microscopy. In both MH-S cell line and mouse peritoneal macrophages stimulated by heat-inactivated BCG, FSF was found to up-regulate autophagy-related proteins microtubule-associated protein 1A/1B-light chain 3 (LC3) and protein 62 (p62), and suppress the induced proinflammatory cytokine TNF-α, CCL5, and IL-6. FSF actively modulates immune processes through suppressing BCG-mediated inflammation by promoting autophagy in MH-S cells and mouse peritoneal macrophages. We suggest that FSF may be useful as an adjunctive therapeutic agent for TB infection by modulating cell survival through autophagy and reducing inflammation.
Collapse
Affiliation(s)
- Lea Ling-Yu Kan
- Institute of Chinese Medicine and State Key Laboratory of Research on Bioactivities and Clinical Applications of Medicinal Plants, The Chinese University of Hong Kong, Hong Kong, China
| | - Dehua Liu
- Institute of Chinese Medicine and State Key Laboratory of Research on Bioactivities and Clinical Applications of Medicinal Plants, The Chinese University of Hong Kong, Hong Kong, China
| | - Ben Chung-Lap Chan
- Institute of Chinese Medicine and State Key Laboratory of Research on Bioactivities and Clinical Applications of Medicinal Plants, The Chinese University of Hong Kong, Hong Kong, China
| | - Miranda Sin-Man Tsang
- Institute of Chinese Medicine and State Key Laboratory of Research on Bioactivities and Clinical Applications of Medicinal Plants, The Chinese University of Hong Kong, Hong Kong, China.,Department of Chemical Pathology, The Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong, China
| | - Tianheng Hou
- Department of Chemical Pathology, The Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong, China
| | - Ping Chung Leung
- Institute of Chinese Medicine and State Key Laboratory of Research on Bioactivities and Clinical Applications of Medicinal Plants, The Chinese University of Hong Kong, Hong Kong, China
| | - Christopher Wai-Kei Lam
- Faculty of Medicine and State Key Laboratory of Quality Research in Chinese Medicines, Macau University of Science and Technology, Macau, China
| | - Chun Kwok Wong
- Institute of Chinese Medicine and State Key Laboratory of Research on Bioactivities and Clinical Applications of Medicinal Plants, The Chinese University of Hong Kong, Hong Kong, China.,Department of Chemical Pathology, The Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong, China.,Li Dak Sum Yip Yio Chin R & D Centre for Chinese Medicine, The Chinese University of Hong Kong, Hong Kong, China
| |
Collapse
|
|
20
|
McDowell SH, Gallaher SA, Burden RE, Scott CJ. Leading the invasion: The role of Cathepsin S in the tumour microenvironment. BIOCHIMICA ET BIOPHYSICA ACTA-MOLECULAR CELL RESEARCH 2020; 1867:118781. [PMID: 32544418 DOI: 10.1016/j.bbamcr.2020.118781] [Citation(s) in RCA: 21] [Impact Index Per Article: 4.2] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Subscribe] [Scholar Register] [Received: 01/02/2020] [Revised: 05/31/2020] [Accepted: 06/04/2020] [Indexed: 02/07/2023]
Abstract
Elevated expression of the cysteine protease Cathepsin S has been correlated with a number of different cancer types in recent years. As tools have been developed to enable more accurate examination of individual cathepsin species, our knowledge and appreciation of the role that this protease plays in facilitating cancer has increased exponentially. This review focuses on our current understanding of the role of Cathepsin S within tumours and the surrounding microenvironment. While various publications have shown that Cathepsin S can be derived from tumour cells themselves, a plethora of more recent studies have identified that Cathepsin S can also be derived from other cell types within the tumour microenvironment including endothelial cells, macrophages and T cells. Furthermore, specific proteolytic substrates cleaved by Cathepsin S have also been identified which have reinforced our hypothesis that this protease facilitates key steps within tumours leading to their invasion, angiogenesis and metastasis.
Collapse
Affiliation(s)
- Sara H McDowell
- The Patrick G Johnston Centre for Cancer Research, Medical Biology Centre, Queen's University Belfast, 97 Lisburn Road, Belfast BT9 7AE, UK.
| | - Samantha A Gallaher
- The Patrick G Johnston Centre for Cancer Research, Medical Biology Centre, Queen's University Belfast, 97 Lisburn Road, Belfast BT9 7AE, UK.
| | - Roberta E Burden
- School of Pharmacy, Medical Biology Centre, Queen's University Belfast, 97 Lisburn Road, Belfast BT9 7BL, UK.
| | - Christopher J Scott
- The Patrick G Johnston Centre for Cancer Research, Medical Biology Centre, Queen's University Belfast, 97 Lisburn Road, Belfast BT9 7AE, UK.
| |
Collapse
|
|
21
|
Lin CL, Hung TW, Ying TH, Lin CJ, Hsieh YH, Chen CM. Praeruptorin B Mitigates the Metastatic Ability of Human Renal Carcinoma Cells through Targeting CTSC and CTSV Expression. Int J Mol Sci 2020; 21:ijms21082919. [PMID: 32331211 PMCID: PMC7216260 DOI: 10.3390/ijms21082919] [Citation(s) in RCA: 7] [Impact Index Per Article: 1.4] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/04/2020] [Revised: 04/19/2020] [Accepted: 04/21/2020] [Indexed: 12/14/2022] Open
Abstract
Renal cell carcinoma (RCC) is the most common adult kidney cancer, and accounts for 85% of all cases of kidney cancers worldwide. Praeruptorin B (Pra-B) is a bioactive constituent of Peucedanum praeruptorum Dunn and exhibits several pharmacological activities, including potent antitumor effects. However, the anti-RCC effects of Pra-B and their underlying mechanisms are unclear; therefore, we explored the effects of Pra-B on RCC cells in this study. We found that Pra-B nonsignificantly influenced the cell viability of human RCC cell lines 786-O and ACHN at a dose of less than 30 μM for 24 h treatment. Further study revealed that Pra-B potently inhibited the migration and invasion of 786-O and ACHN cells, as well as downregulated the mRNA and protein expression of cathepsin C (CTSC) and cathepsin V (CTSV) of 786-O and ACHN cells. Mechanistically, Pra-B also reduced the protein levels of phospho (p)-epidermal growth factor receptor (EGFR), p-mitogen-activated protein kinase kinase (MEK), and p-extracellular signal-regulated kinases (ERK) in RCC cells. In addition, Pra-B treatment inhibited the effect of EGF on the upregulation of EGFR–MEK–ERK, CTSC and CTSV expression, cellular migration, and invasion of 786-O cells. Our findings are the first to demonstrate that Pra-B can reduce the migration and invasion ability of human RCC cells through suppressing the EGFR-MEK-ERK signaling pathway and subsequently downregulating CTSC and CTSV. This evidence suggests that Pra-B can be developed as an effective antimetastatic agent for the treatment of RCC.
Collapse
Affiliation(s)
- Chia-Liang Lin
- Institute of Biochemistry, Microbiology and Immunology, Chung Shan Medical University, Taichung 40201, Taiwan; (C.-L.L.); (C.-J.L.)
- Department of Medicine, Mackay Medical College, New Taipei City 252, Taiwan
| | - Tung-Wei Hung
- Division of Nephrology, Department of Medicine, Chung Shan Medical University Hospital, Taichung 40201, Taiwan;
- School of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan
| | - Tsung-Ho Ying
- Department of Obstetrics and Gynecology, Chung Shan Medical University Hospital, Taichung 40201, Taiwan;
- Department of Obstetrics and Gynecology, School of Medicine, College of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan
| | - Chi-Jui Lin
- Institute of Biochemistry, Microbiology and Immunology, Chung Shan Medical University, Taichung 40201, Taiwan; (C.-L.L.); (C.-J.L.)
| | - Yi-Hsien Hsieh
- Institute of Biochemistry, Microbiology and Immunology, Chung Shan Medical University, Taichung 40201, Taiwan; (C.-L.L.); (C.-J.L.)
- Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan
- Clinical Laboratory, Chung Shan Medical University Hospital, Taichung 40201, Taiwan
- Correspondence: (Y.-H.H.); (C.-M.C.); Tel.: +886-04-24730022 (Y.-H.H.); Fax: +886-04-23248110 (Y.-H.H.)
| | - Chien-Min Chen
- Division of Neurosurgery, Department of Surgery, Changhua Christian Hospital, Changhua 50006, Taiwan
- School of Medicine, Kaohsiung Medical University, Kaohsiung 80708, Taiwan
- College of Nursing and Health Sciences, Dayeh University, Changhua 51591, Taiwan
- Correspondence: (Y.-H.H.); (C.-M.C.); Tel.: +886-04-24730022 (Y.-H.H.); Fax: +886-04-23248110 (Y.-H.H.)
| |
Collapse
|
|
22
|
Biray Avci C, Sezgin B, Goker Bagca B, Karci HB, Gode S. PI3K/AKT/mTOR pathway and autophagy regulator genes in paranasal squamous cell carcinoma metastasis. Mol Biol Rep 2020; 47:3641-3651. [PMID: 32319010 DOI: 10.1007/s11033-020-05458-8] [Citation(s) in RCA: 5] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/21/2020] [Accepted: 04/11/2020] [Indexed: 12/19/2022]
Abstract
Although there are many studies on the role of PI3K/AKT/mTOR pathway and autophagy genes in the mechanism of head and neck cancer formation and prognostic significance, there is no study investigating the role of the genes in paranasal sinus carcinomas. The aim of the study was to assess the role of the PI3K/AKT/mTOR pathway and autophagy related gene expression changes in squamous cell carcinoma of paranasal sinuses with and without neck metastasis. Eight paranasal squamous cell carcinoma patients (five without and three with neck metastasis) were included. Tissues were obtained during the surgery. Total RNA was isolated from the tissues and cDNA synthesis was performed. Expression levels of the genes were determined using qRT-PCR method. The results were evaluated using the 2-∆∆Ct method, and fold changes of the gene expression levels in primary tumor and neck metastasis tissues were calculated according to the normal tissue. Expression levels of both PI3K/AKT/mTOR pathway and positive regulators of autophagy were significantly increased in metastasis-related two groups, especially in neck metastasis tissues. The increase in PI3K/AKT/mTOR pathway and autophagy related gene expression levels may support the metastatic character in paranasal squamous cell carcinomas. This is the first study to assess autophagy related genes in paranasal sinus cancer at transcriptome-level. Support of the transcriptome-level findings by the further protein analyses will contribute to the illumination of the rare paranasal sinus cancer molecular biology.
Collapse
Affiliation(s)
- Cigir Biray Avci
- Medical Biology Department, Ege University School of Medicine, Bornova, 35100, Izmir, Turkey
| | - Baha Sezgin
- Otorhinolaryngology Department, Ege University School of Medicine, Izmir, Turkey
| | - Bakiye Goker Bagca
- Medical Biology Department, Ege University School of Medicine, Bornova, 35100, Izmir, Turkey.
| | - Halil Bulent Karci
- Otorhinolaryngology Department, Ege University School of Medicine, Izmir, Turkey
| | - Sercan Gode
- Otorhinolaryngology Department, Ege University School of Medicine, Izmir, Turkey
| |
Collapse
|
|
23
|
Xu Z, Han X, Ou D, Liu T, Li Z, Jiang G, Liu J, Zhang J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl Microbiol Biotechnol 2019; 104:575-587. [PMID: 31832711 DOI: 10.1007/s00253-019-10257-8] [Citation(s) in RCA: 393] [Impact Index Per Article: 65.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/02/2019] [Revised: 11/05/2019] [Accepted: 11/12/2019] [Indexed: 12/11/2022]
Abstract
Autophagy is a highly conserved catabolic process and participates in a variety of cellular biological activities. The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway, as a critical regulator of autophagy, is involved in the initiation and promotion of a series of pathological disorders including various tumors. Autophagy also participates in regulating the balance between the tumor and the tumor microenvironment. Natural products have been considered a treasure of new drug discoveries and are of great value to medicine. Mounting evidence has suggested that numerous natural products are targeting PI3K/AKT/mTOR-mediated autophagy, thereby suppressing tumor growth. Furthermore, autophagy plays a "double-edged sword" role in different tumors. Targeting PI3K/AKT/mTOR-mediated autophagy is an important therapeutic strategy for a variety of tumors, and plays important roles in enhancing the chemosensitivity of tumor cells and avoiding drug resistance. Therefore, we summarized the roles of PI3K/AKT/mTOR-mediated autophagy in tumorigenesis, progression, and drug resistance of tumors, which may be utilized to design preferably therapeutic strategies for various tumors.
Collapse
Affiliation(s)
- Zhenru Xu
- Department of Rheumatology, The First Affiliated Hospital of University of South China, Hengyang, Hunan, China
| | - Xu Han
- Molecular Biology Research Center & Center for Medical Genetics, School of Life Sciences, Central South University, Changsha, Hunan, China
| | - Daming Ou
- Department of Rheumatology, The First Affiliated Hospital of University of South China, Hengyang, Hunan, China
| | - Ting Liu
- Department of Rheumatology, The First Affiliated Hospital of University of South China, Hengyang, Hunan, China
| | - Zunxiong Li
- University of South China, Hengyang, Hunan, China
| | - Guanmin Jiang
- Department of Clinical Laboratory, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong, China
| | - Jing Liu
- Molecular Biology Research Center & Center for Medical Genetics, School of Life Sciences, Central South University, Changsha, Hunan, China.
| | - Ji Zhang
- Department of Rheumatology, The First Affiliated Hospital of University of South China, Hengyang, Hunan, China.
| |
Collapse
|
|
24
|
Wang H, Liu Y, Wang D, Xu Y, Dong R, Yang Y, Lv Q, Chen X, Zhang Z. The Upstream Pathway of mTOR-Mediated Autophagy in Liver Diseases. Cells 2019; 8:E1597. [PMID: 31835352 PMCID: PMC6953127 DOI: 10.3390/cells8121597] [Citation(s) in RCA: 177] [Impact Index Per Article: 29.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/11/2019] [Revised: 11/29/2019] [Accepted: 12/03/2019] [Indexed: 12/11/2022] Open
Abstract
Autophagy, originally found in liver experiments, is a cellular process that degrades damaged organelle or protein aggregation. This process frees cells from various stress states is a cell survival mechanism under stress stimulation. It is now known that dysregulation of autophagy can cause many liver diseases. Therefore, how to properly regulate autophagy is the key to the treatment of liver injury. mechanistic target of rapamycin (mTOR)is the core hub regulating autophagy, which is subject to different upstream signaling pathways to regulate autophagy. This review summarizes three upstream pathways of mTOR: the phosphoinositide 3-kinase (PI3K)/protein kinase (AKT) signaling pathway, the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway, and the rat sarcoma (Ras)/rapidly accelerated fibrosarcoma (Raf)/mitogen-extracellular activated protein kinase kinase (MEK)/ extracellular-signal-regulated kinase (ERK) signaling pathway, specifically explored their role in liver fibrosis, hepatitis B, non-alcoholic fatty liver, liver cancer, hepatic ischemia reperfusion and other liver diseases through the regulation of mTOR-mediated autophagy. Moreover, we also analyzed the crosstalk between these three pathways, aiming to find new targets for the treatment of human liver disease based on autophagy.
Collapse
Affiliation(s)
- Haojie Wang
- College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471000, China; (H.W.); (Y.X.); (R.D.); (Y.Y.); (Q.L.); (X.C.)
| | - Yumei Liu
- College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471000, China; (H.W.); (Y.X.); (R.D.); (Y.Y.); (Q.L.); (X.C.)
| | - Dongmei Wang
- College of Medical, Henan University of Science and Technology, Luoyang 471000, China;
| | - Yaolu Xu
- College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471000, China; (H.W.); (Y.X.); (R.D.); (Y.Y.); (Q.L.); (X.C.)
| | - Ruiqi Dong
- College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471000, China; (H.W.); (Y.X.); (R.D.); (Y.Y.); (Q.L.); (X.C.)
| | - Yuxiang Yang
- College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471000, China; (H.W.); (Y.X.); (R.D.); (Y.Y.); (Q.L.); (X.C.)
| | - Qiongxia Lv
- College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471000, China; (H.W.); (Y.X.); (R.D.); (Y.Y.); (Q.L.); (X.C.)
| | - Xiaoguang Chen
- College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471000, China; (H.W.); (Y.X.); (R.D.); (Y.Y.); (Q.L.); (X.C.)
| | - Ziqiang Zhang
- College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471000, China; (H.W.); (Y.X.); (R.D.); (Y.Y.); (Q.L.); (X.C.)
| |
Collapse
|
|
25
|
Endothelial cell-derived small extracellular vesicles suppress cutaneous wound healing through regulating fibroblasts autophagy. Clin Sci (Lond) 2019; 133:CS20190008. [PMID: 30988132 DOI: 10.1042/cs20190008] [Citation(s) in RCA: 27] [Impact Index Per Article: 4.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/04/2019] [Revised: 03/27/2019] [Accepted: 04/15/2019] [Indexed: 02/06/2023]
Abstract
Diabetic foot ulcer is a life-threatening clinical problem in diabetic patients. Endothelial cell-derived small extracellular vesicles (sEVs) are important mediators of intercellular communication in the pathogenesis of several diseases. However, the exact mechanisms of wound healing mediated by endothelial cell-derived sEVs remain unclear. sEVs were isolated from human umbilical vein endothelial cells (HUVECs) pretreated with or without advanced glycation end products (AGEs). The roles of HUVEC-derived sEVs on the biological characteristics of skin fibroblasts were investigated both in vitro and in vivo We demonstrate that sEVs derived from AGEs-pretreated HUVECs (AGEs-sEVs) could inhibit collagen synthesis by activating autophagy of human skin fibroblasts. Additionally, treatment with AGEs-sEVs could delay the wound healing process in Sprague-Dawley (SD) rats. Further analysis indicated that miR-106b-5p was up-regulated in AGEs-sEVs and importantly, in exudate-derived sEVs from patients with diabetic foot ulcer. Consequently, sEV-mediated uptake of miR-106b-5p in recipient fibroblasts reduces expression of extracellular signal-regulated kinase 1/2 (ERK1/2), resulting in fibroblasts autophagy activation and subsequent collagen degradation. Collectively, our data demonstrate that miR-106b-5p could be enriched in AGEs-sEVs, then decreases collagen synthesis and delays cutaneous wound healing by triggering fibroblasts autophagy through reducing ERK1/2 expression.
Collapse
|
|
26
|
Abstract
Cathepsins (CTS) are mainly lysosomal acid hydrolases extensively involved in the prognosis of different diseases, and having a distinct role in tumor progression by regulating cell proliferation, autophagy, angiogenesis, invasion, and metastasis. As all these processes conjunctively lead to cancer progression, their site-specific regulation might be beneficial for cancer treatment. CTS regulate activation of the proteolytic cascade and protein turnover, while extracellular CTS is involved in promoting extracellular matrix degradation and angiogenesis, thereby stimulating invasion and metastasis. Despite cancer regulation, the involvement of CTS in cellular adaptation toward chemotherapy and radiotherapy augments their therapeutic potential. However, lysosomal permeabilization mediated cytosolic translocation of CTS induces programmed cell death. This complex behavior of CTS generates the need to discuss the different aspects of CTS associated with cancer regulation. In this review, we mainly focused on the significance of each cathepsin in cancer signaling and their targeting which would provide noteworthy information in the context of cancer biology and therapeutics.
Collapse
Affiliation(s)
- Tejinder Pal Khaket
- Department of Biotechnology, Daegu University, Gyeongsan, Gyeongbuk 38453, Republic of Korea
| | - Taeg Kyu Kwon
- Department of Immunology, School of Medicine, Keimyung University, Dalseo-Gu, Daegu 704-701, Republic of Korea.
| | - Sun Chul Kang
- Department of Biotechnology, Daegu University, Gyeongsan, Gyeongbuk 38453, Republic of Korea.
| |
Collapse
|
|
27
|
Jiang M, Li Z, Zhu G. The role of autophagy in the pathogenesis of periodontal disease. Oral Dis 2019; 26:259-269. [PMID: 30674085 DOI: 10.1111/odi.13045] [Citation(s) in RCA: 20] [Impact Index Per Article: 3.3] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/07/2018] [Revised: 01/13/2019] [Accepted: 01/16/2019] [Indexed: 12/16/2022]
Affiliation(s)
- Ming Jiang
- Department of Stomatology, Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology Wuhan China
| | - Zhuoneng Li
- Centers for Disease Control and Prevention of Wuhan Wuhan China
| | - Guangxun Zhu
- Department of Stomatology, Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology Wuhan China
| |
Collapse
|
|
28
|
Chiang PJ, Wu SM, Tseng MJ, Huang PJ. Automated Bright Field Segmentation of Cells and Vacuoles Using Image Processing Technique. Cytometry A 2018; 93:1004-1018. [PMID: 30230197 DOI: 10.1002/cyto.a.23595] [Citation(s) in RCA: 7] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/18/2017] [Revised: 07/18/2018] [Accepted: 07/30/2018] [Indexed: 12/19/2022]
Abstract
Understanding the mechanisms and other variants of programmed cell death will help provide deeper insight into various disease processes. Although complex procedures are required to distinguish each type of cell death, the formation of vacuoles is one of the important features in some process of cell death under different conditions. Thus, monitoring and counting the number of vacuoles and the ratio of cells with vacuoles is a commonly used method to indicate and quantify the efficacy of the therapy. Several studies have shown that image processing can provide a quick, convenient and precise mean of performing cell detection. Hence, this study uses an image processing technique to detect and quantify vacuolated cells without the need for dyes. The system both counts the number of vacuolated cells and determines the ratio of cells with vacuoles. The performance of the proposed image processing system was evaluated using 38 images. It has been shown that a strong correlation exists between the automated counts and the manual counts. Furthermore, the absolute percentage errors between automated counts and manual counts for cell detection and vacuolated cell detection using data pooled from all images are 3.61 and 3.33%, respectively. A user-friendly graphical user interface (GUI) is also developed and freely available for download, providing researchers in biomedicine with a more convenient instrument for vacuolization analysis.
Collapse
Affiliation(s)
- Pei-Ju Chiang
- Department of Mechanical Engineering and Advanced Institute of Manufacturing with High-Tech Innovations, National Chung Cheng University, Chia-Yi, Taiwan, ROC
| | - Shao-Ming Wu
- Department of Mechanical Engineering and Advanced Institute of Manufacturing with High-Tech Innovations, National Chung Cheng University, Chia-Yi, Taiwan, ROC
| | - Min-Jen Tseng
- Department of Biomedical Sciences and Institute of Molecular Biology, National Chung Cheng University, Chia-Yi, Taiwan, ROC
| | - Pin-Jie Huang
- Department of Biomedical Sciences and Institute of Molecular Biology, National Chung Cheng University, Chia-Yi, Taiwan, ROC
| |
Collapse
|
|
29
|
Down-regulation of cathepsin S and matrix metalloproteinase-9 via Src, a non-receptor tyrosine kinase, suppresses triple-negative breast cancer growth and metastasis. Exp Mol Med 2018; 50:1-14. [PMID: 30185799 PMCID: PMC6123788 DOI: 10.1038/s12276-018-0135-9] [Citation(s) in RCA: 33] [Impact Index Per Article: 4.7] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/19/2018] [Accepted: 05/28/2018] [Indexed: 02/07/2023] Open
Abstract
Triple-negative breast cancer (TNBC) is a highly metastatic breast cancer with poor prognosis. In the present study, we demonstrated that Src, a non-receptor tyrosine kinase, might provide an effective therapeutic strategy to overcome TNBC invasion and metastasis, which are mediated via the synergistic action of the lysosomal enzyme cathepsin S (CTSS) and gelatinase MMP-9. Knock-down of MMP-9 and CTSS using siRNAs resulted in a synergistic suppression of MDA-MB-231 cell invasion, which was similarly observed with pharmacological inhibitors. During the screening of new drug candidates that suppress both CTSS and MMP-9, BJ-2302, a novel 7-azaindolin-2-one derivative, was discovered. Src, an upstream activator of both pathways (PI3K/Akt and Ras/Raf/ERK) responsible for the expression of CTSS and MMP-9, was identified as a high-affinity target of BJ-2302 (IC90: 3.23 µM) through a Src kinase assay and a drug affinity responsive target stability (DARTS) assay. BJ-2302 effectively suppressed MDA-MB-231 cell invasion (Matrigel invasion assay) and metastasis (chorioallantoic membrane assay xenografted with MDA-MB-231-luc2-tdTomato cancer cells). Unlike Z-FL-COCHO (potent CTSS inhibitor), BJ-2302 did not induce any cytotoxicity in MCF-10A normal breast epithelial cells. Additionally, BJ-2302 (1 mg/kg) strongly suppressed TNBC cell proliferation in vitro and tumor growth in a xenograft mouse tumor model. The anti-metastatic and anti-tumor effects of BJ-2302 were superior to those of Z-FL-COCHO (1 mg/kg) or batimastat (30 mg/kg), a pan-MMP inhibitor. In summary, inhibition of Src kinase suppressed TNBC tumor growth and metastasis, and Src inhibitors such as BJ-2302 may constitute a novel therapeutic tool to treat breast cancer that expresses high levels of CTSS and MMP-9.
Collapse
|
|
30
|
Z-FL-COCHO, a cathepsin S inhibitor, enhances oxaliplatin-mediated apoptosis through the induction of endoplasmic reticulum stress. Exp Mol Med 2018; 50:1-11. [PMID: 30120227 PMCID: PMC6098103 DOI: 10.1038/s12276-018-0138-6] [Citation(s) in RCA: 15] [Impact Index Per Article: 2.1] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/08/2018] [Revised: 05/28/2018] [Accepted: 05/30/2018] [Indexed: 02/08/2023] Open
Abstract
Multiple cancer cells highly express cathepsin S, which has pro-tumoral effects. However, it was previously unknown whether knockdown or a pharmacological inhibitor (ZFL) of cathepsin S acts as an inducer of ER stress. Here, ZFL and knockdown of cathepsin S markedly induced ER stress through the up-regulation of calcium levels in the cytosol. Induction of calcium levels by inhibition of cathepsin S is markedly blocked by an inhibitor of the IP3 receptor and the ryanodine receptor Ca2+ channel in the ER, but an inhibitor of a mitochondrial Ca2+ uniporter had no effect on ZFL-induced calcium levels. Furthermore, production of mitochondrial ROS by ZFL was associated with an increase in cytosolic calcium levels. ZFL-mediated ER stress enhanced anti-cancer drug-induced apoptotic cell death, and pretreatment with chemical chaperones or down-regulation of ATF4 and CHOP by small interfering RNA markedly reduced ZFL plus oxaliplatin-induced apoptosis. Taken together, our findings reveal that inhibition of cathepsin S is an inducer of ER stress; these findings may contribute to the enhancement of therapeutic efficiency in cancer cells. A drug that inhibits a key cancer enzyme could be used in combination with anti-cancer drugs to improve sensitivity to treatment. The intracellular endoplasmic reticulum (ER) is involved in several vital processes in cells, including folding and processing proteins. Taeg Kyu Kwon at Keimyung University, Daegu, South Korea, and co-workers have demonstrated how inhibition of cathepsin S, which is expressed in many cancer cells, induces ER stress. In trials on human kidney cancer cells grafted onto mice and in vitro, the team found that ZFL (cathepsin S inhibitor) triggered transient ER stress by increasing calcium levels inside cells. Subsequent treatment with the anti-cancer drug oxaliplatin resulted in increased cancer cell death.
Collapse
|
|
31
|
Tan J, Qian X, Song B, An X, Cai T, Zuo Z, Ding D, Lu Y, Li H. Integrated bioinformatics analysis reveals that the expression of cathepsin S is associated with lymph node metastasis and poor prognosis in papillary thyroid cancer. Oncol Rep 2018; 40:111-122. [PMID: 29749483 PMCID: PMC6059735 DOI: 10.3892/or.2018.6428] [Citation(s) in RCA: 15] [Impact Index Per Article: 2.1] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/30/2017] [Accepted: 04/26/2018] [Indexed: 12/20/2022] Open
Abstract
The prognosis of the majority of patients with papillary thyroid cancer (PTC) is excellent, although there are patients who experience disease recurrence and progression. The aim of the present study was to identify potential prognostic risk markers in PTC. Differentially expressed genes (DEGs), identified from four Genome Expression Omnibus cohorts were subjected to functional enrichment analyses with Gene Ontology terms and the Kyoto Encyclopedia of Genes and Genome pathways. Hub genes, filtered from cytoHubba, were validated using the The Cancer Genome Atlas (TCGA) cohort, and their associations with clinicopathological features and prognosis were analyzed. A total of 277 DEGs were identified following data preprocessing. DEGs were primarily enriched in 'small cell lung cancer', 'ECM-receptor interaction', 'pathways in cancer'and 'tyrosine metabolism'. Hub genes [APOE, cathepsin S (CTSS), insulin receptor substrate 1 (IRS1), KIT, LGALS3, RUNX2 and TGFBR1] were extracted from cytoHubba. Their expression in the TCGA cohort was consistent with that in the GEO cohorts. CTSS (P=0.006) and IRS1 (P=0.005) were associated with disease‑free survival, as determined using the Kaplan-Meier analysis. CTSS was an independent risk factor for poor disease‑free survival (HR, 2.649; 95% CI, 1.095-6.409; P=0.031). Patients with high expression of CTSS exhibited different histological types (increased tall-cell subtype and reduced follicular subtype; P<0.001), more frequent lymph node metastasis (P<0.001) and advanced tumor-node-metastasis stages (P=0.049) compared with the low-expression group. High expression of CTSS was independently associated with lymph node metastasis (OR, 2.015; 95% CI, 1.225-3.315; P=0.006). Therefore, CTSS may serve as a predictive risk marker for the progression and prognosis of PTC.
Collapse
Affiliation(s)
- Juan Tan
- Department of Endocrinology, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 211166, P.R. China
- Department of Gerontology, The Affiliated Huaian No. 1 People's Hospital of Nanjing Medical University, Huai'an, Jiangsu 223300, P.R. China
| | - Xiaoxiao Qian
- Department of Endocrinology, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 211166, P.R. China
| | - Bin Song
- Department of Endocrinology, Clinical Medical College of Yangzhou University, Yangzhou, Jiangsu 225001, P.R. China
| | - Xiumin An
- Department of Endocrinology, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 211166, P.R. China
| | - Tingting Cai
- Department of Endocrinology, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 211166, P.R. China
| | - Zhihua Zuo
- Department of Endocrinology, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 211166, P.R. China
| | - Dafa Ding
- Department of Endocrinology, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 211166, P.R. China
| | - Yibing Lu
- Department of Endocrinology, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 211166, P.R. China
| | - Hong Li
- Medical Examination Center, The Affiliated Huaian No. 1 People's Hospital of Nanjing Medical University, Huai'an, Jiangsu 223300, P.R. China
| |
Collapse
|
|
32
|
Cysteine cathepsins as a prospective target for anticancer therapies-current progress and prospects. Biochimie 2018; 151:85-106. [PMID: 29870804 DOI: 10.1016/j.biochi.2018.05.023] [Citation(s) in RCA: 27] [Impact Index Per Article: 3.9] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/19/2017] [Accepted: 05/31/2018] [Indexed: 02/08/2023]
Abstract
Cysteine cathepsins (CTS), being involved in both physiological and pathological processes, play an important role in the human body. During the last 30 years, it has been shown that CTS are highly upregulated in a wide variety of cancer types although they have received a little attention as a potential therapeutic target as compared to serine or metalloproteinases. Studies on the increasing problem of neoplastic progression have revealed that secretion of cell-surface- and intracellular cysteine proteases is aberrant in tumor cells and has an impact on their growth, invasion, and metastasis by taking part in tumor angiogenesis, in apoptosis, and in events of inflammatory and immune responses. Considering the role of CTS in carcinogenesis, inhibition of these enzymes becomes an attractive strategy for cancer therapy. The downregulation of natural CTS inhibitors (CTSsis), such as cystatins, observed in various types of cancer, supports this claim. The intention of this review is to highlight the relationship of CTS with cancer and to present illustrations that explain how some of their inhibitors affect processes related to neoplastic progression.
Collapse
|
|
33
|
Sooro MA, Zhang N, Zhang P. Targeting EGFR-mediated autophagy as a potential strategy for cancer therapy. Int J Cancer 2018; 143:2116-2125. [DOI: 10.1002/ijc.31398] [Citation(s) in RCA: 51] [Impact Index Per Article: 7.3] [Reference Citation Analysis] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/11/2017] [Revised: 03/05/2018] [Accepted: 03/12/2018] [Indexed: 12/19/2022]
Affiliation(s)
- Mopa Alina Sooro
- Jiangsu Key Laboratory of New Drug Screening; China Pharmaceutical University; Nanjing 210009 China
| | - Ni Zhang
- Jiangsu Key Laboratory of New Drug Screening; China Pharmaceutical University; Nanjing 210009 China
| | - Pinghu Zhang
- Medical College, Institute of Translational Medicine, Yangzhou University; Yangzhou 225001 China
- Jiangsu Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Treatment of Senile Diseases; Medical College, Yangzhou University; Yangzhou 225001 China
| |
Collapse
|
|
34
|
Chen CT, Hsieh MJ, Hsieh YH, Hsin MC, Chuang YT, Yang SF, Yang JS, Lin CW. Sulforaphane suppresses oral cancer cell migration by regulating cathepsin S expression. Oncotarget 2018; 9:17564-17575. [PMID: 29707130 PMCID: PMC5915138 DOI: 10.18632/oncotarget.24786] [Citation(s) in RCA: 10] [Impact Index Per Article: 1.4] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/09/2017] [Accepted: 02/28/2018] [Indexed: 12/30/2022] Open
Abstract
Sulforaphane has been demonstrated to exert numerous biological effects, such as neuroprotective, anti-inflammatory, and anticancer effects. However, the detailed effects of sulforaphane on human oral cancer cell migration and the underlying mechanisms remain unclear. In this study, we observed that sulforaphane attenuated SCC-9 and SCC-14 cell motility and invasiveness by reducing cathepsin S expression. Moreover, sulforaphane increased microtubule-associated protein 1 light chain 3 (LC3) conversion, and the knockdown of LC3 by siRNA increased cell migration ability. Regarding the mechanism, sulforaphane inhibited the cell motility of oral cancer cells through the extracellular signal-regulated kinase (ERK) pathway, which in turn reversed cell motility. In conclusion, sulforaphane suppress cathepsin S expression by inducing autophage through ERK signaling pathway. Thus, cathepsin S and LC3 may be new targets for oral cancer treatment.
Collapse
Affiliation(s)
- Chang-Tai Chen
- Institute of Oral Sciences, Chung Shan Medical University, Taichung, Taiwan
- School of Dentistry, Chung Shan Medical University, Taichung, Taiwan
| | - Ming-Ju Hsieh
- Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- Cancer Research Center, Changhua Christian Hospital, Changhua, Taiwan
- Graduate Institute of Biomedical Sciences, China Medical University, Taichung, Taiwan
| | - Yi-Hsien Hsieh
- Institute of Biochemistry, Microbiology and Immunology, Chung Shan Medical University, Taichung, Taiwan
| | - Min-Chieh Hsin
- Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
| | - Yi-Ting Chuang
- Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
| | - Shun-Fa Yang
- Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
| | - Jia-Sin Yang
- Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
| | - Chiao-Wen Lin
- Institute of Oral Sciences, Chung Shan Medical University, Taichung, Taiwan
- School of Dentistry, Chung Shan Medical University, Taichung, Taiwan
- Department of Dentistry, Chung Shan Medical University Hospital, Taichung, Taiwan
| |
Collapse
|
|
35
|
Targeting of cathepsin C induces autophagic dysregulation that directs ER stress mediated cellular cytotoxicity in colorectal cancer cells. Cell Signal 2018; 46:92-102. [PMID: 29501728 DOI: 10.1016/j.cellsig.2018.02.017] [Citation(s) in RCA: 24] [Impact Index Per Article: 3.4] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/04/2018] [Revised: 02/28/2018] [Accepted: 02/28/2018] [Indexed: 12/14/2022]
Abstract
As Autophagy is a pivotal mechanism of cancer cell survival and the development of chemotherapeutic resistance; therefore, new approaches are warranted for its targeting which may be fulfilled by cathepsins regulation. Amongst cathepsins, cathepsin C (CTSC) is highly expressed in various cancers and possesses significant therapeutic potential in autoimmune disorders; however, its role in colorectal cancer has not been explored. Herein, we aimed to investigate the role of CTSC in autophagy regulation mediated colorectal carcinoma cell proliferation. Cathepsin C targeting through inhibitors/siRNA leads to the accumulation of light chain 3 II and p62 without affecting the lysosomal integrity, revealed dysfunctional autolysosomal degradation which is also substantiated by proteolytic studies. Cathepsin C inhibition showed comparable autophagy blockade with E64d and augmented the autophagy blockade mediated by bafilomycin. Loss of CTSC function also induced ER stress-mediated JNK phosphorylation accompanied by the translocation of mitochondrial cyt c followed by apoptotic cell death in colorectal carcinoma cells. Taken together, the study reveals that CTSC targeting plays a key role in the regulation of autophagy mediated colorectal cancer cell proliferation. Further investigations are required to determine the functional role of CTSC in other tumors also which may have implications for the therapeutic prevention of cancer in the future.
Collapse
|
|
36
|
Yao D, Wang P, Zhang J, Fu L, Ouyang L, Wang J. Deconvoluting the relationships between autophagy and metastasis for potential cancer therapy. Apoptosis 2018; 21:683-98. [PMID: 27003389 DOI: 10.1007/s10495-016-1237-2] [Citation(s) in RCA: 21] [Impact Index Per Article: 3.0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 02/05/2023]
Abstract
Autophagy is a highly conserved lysosome-dependent degradation process that may digest some long-lived proteins and damaged organelles. As an essential homeostasis maintaining system in normal cells, autophagy plays a key role in several pathological settings, especially cancer. Metastasis, known as a crucial hallmark of cancer progression, is the primary cause of cancer lethality. The role of autophagy in metastasis is quite complex as supportive evidence has indicated both pro-metastatic and anti-metastatic functions of autophagy. Autophagy can inhibit metastasis by restricting necrosis and mediating autophagic cell death, whereas it may also promote metastasis by enhancing cancer cell fitness in response to stress. Moreover, the function of autophagy is context- and stage-dependent. Specifically, during the early steps of metastasis, autophagy mainly serves as a suppressor, while it plays a pro-metastatic role in the later steps. Here, we focus on highlighting the dual roles of autophagy in metastasis and address the molecular mechanisms involved in this process, which may provide a new insight into cancer biology. While, we also summarize several anti-metastatic agents manipulating autophagy, in the hope of shedding light on exploration of potential novel drugs for future cancer therapy.
Collapse
Affiliation(s)
- Dahong Yao
- State Key Laboratory of Biotherapy & Collaborative Innovation Center of Biotherapy, West China Hospital, Sichuan University, Chengdu, 610041, China
- School of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, Shenyang, 110016, China
| | - Peiqi Wang
- State Key Laboratory of Oral Diseases, West China School of Stomatology, Sichuan University, Chengdu, 610041, China
| | - Jin Zhang
- State Key Laboratory of Biotherapy & Collaborative Innovation Center of Biotherapy, West China Hospital, Sichuan University, Chengdu, 610041, China
- School of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, Shenyang, 110016, China
| | - Leilei Fu
- State Key Laboratory of Biotherapy & Collaborative Innovation Center of Biotherapy, West China Hospital, Sichuan University, Chengdu, 610041, China.
| | - Liang Ouyang
- State Key Laboratory of Biotherapy & Collaborative Innovation Center of Biotherapy, West China Hospital, Sichuan University, Chengdu, 610041, China.
| | - Jinhui Wang
- School of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, Shenyang, 110016, China
| |
Collapse
|
|
37
|
Pires D, Bernard EM, Pombo JP, Carmo N, Fialho C, Gutierrez MG, Bettencourt P, Anes E. Mycobacterium tuberculosis Modulates miR-106b-5p to Control Cathepsin S Expression Resulting in Higher Pathogen Survival and Poor T-Cell Activation. Front Immunol 2017; 8:1819. [PMID: 29326705 PMCID: PMC5741618 DOI: 10.3389/fimmu.2017.01819] [Citation(s) in RCA: 44] [Impact Index Per Article: 5.5] [Reference Citation Analysis] [Abstract] [Key Words] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/20/2017] [Accepted: 12/04/2017] [Indexed: 01/20/2023] Open
Abstract
The success of tuberculosis (TB) bacillus, Mycobacterium tuberculosis (Mtb), relies on the ability to survive in host cells and escape to immune surveillance and activation. We recently demonstrated that Mtb manipulation of host lysosomal cathepsins in macrophages leads to decreased enzymatic activity and pathogen survival. In addition, while searching for microRNAs (miRNAs) involved in posttranscriptional gene regulation during mycobacteria infection of human macrophages, we found that selected miRNAs such as miR-106b-5p were specifically upregulated by pathogenic mycobacteria. Here, we show that miR-106b-5p is actively manipulated by Mtb to ensure its survival in macrophages. Using an in silico prediction approach, we identified miR-106b-5p with a potential binding to the 3'-untranslated region of cathepsin S (CtsS) mRNA. We demonstrated by luminescence-based methods that miR-106b-5p indeed targets CTSS mRNA resulting in protein translation silencing. Moreover, miR-106b-5p gain-of-function experiments lead to a decreased CtsS expression favoring Mtb intracellular survival. By contrast, miR-106b-5p loss-of-function in infected cells was concomitant with increased CtsS expression, with significant intracellular killing of Mtb and T-cell activation. Modulation of miR-106b-5p did not impact necrosis, apoptosis or autophagy arguing that miR-106b-5p directly targeted CtsS expression as a way for Mtb to avoid exposure to degradative enzymes in the endocytic pathway. Altogether, our data suggest that manipulation of miR-106b-5p as a potential target for host-directed therapy for Mtb infection.
Collapse
Affiliation(s)
- David Pires
- Host-Pathogen Interactions Unit, Faculty of Pharmacy, Research Institute for Medicines, iMed-ULisboa, Universidade de Lisboa, Lisboa, Portugal
| | - Elliott M. Bernard
- Host-Pathogen Interactions in Tuberculosis Laboratory, The Francis Crick Institute, London, United Kingdom
| | - João Palma Pombo
- Host-Pathogen Interactions Unit, Faculty of Pharmacy, Research Institute for Medicines, iMed-ULisboa, Universidade de Lisboa, Lisboa, Portugal
| | - Nuno Carmo
- Host-Pathogen Interactions Unit, Faculty of Pharmacy, Research Institute for Medicines, iMed-ULisboa, Universidade de Lisboa, Lisboa, Portugal
| | - Catarina Fialho
- Host-Pathogen Interactions Unit, Faculty of Pharmacy, Research Institute for Medicines, iMed-ULisboa, Universidade de Lisboa, Lisboa, Portugal
| | | | - Paulo Bettencourt
- Host-Pathogen Interactions Unit, Faculty of Pharmacy, Research Institute for Medicines, iMed-ULisboa, Universidade de Lisboa, Lisboa, Portugal
| | - Elsa Anes
- Host-Pathogen Interactions Unit, Faculty of Pharmacy, Research Institute for Medicines, iMed-ULisboa, Universidade de Lisboa, Lisboa, Portugal
| |
Collapse
|
|
38
|
Lewis y antigen promotes p27 degradation by regulating ubiquitin-proteasome activity. Oncotarget 2017; 8:110064-110076. [PMID: 29299130 PMCID: PMC5746365 DOI: 10.18632/oncotarget.22617] [Citation(s) in RCA: 6] [Impact Index Per Article: 0.8] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/28/2015] [Accepted: 10/02/2017] [Indexed: 01/12/2023] Open
Abstract
As a tumor-associated carbohydrate antigen, elevated expression of Lewis y promotes the malignant behaviors of tumor cells. Although our preliminary study showed that the increased expression of Lewis y antigen decreased the expression of cell cycle inhibitor protein p27, the relevant mechanism remains unclear. Autophagy and the ubiquitin-proteasome system are two main ways of intracellular protein degradation, whose abnormal activities are closely associated with progression of malignant tumors. In our present study, we constructed two stable transfected cell lines with high expression of Lewis y antigen, named CAOV3-FUT1 and SKOV3-FUT1. We showed that the proportion of cells at S phase was significantly increased after FUT1 transfection, whereas p27 protein was obviously decreased. The autophagy activity, the levels of ubiquitination, and chymotrypsin-like protease activity were increased remarkably in the transfected cells. Interestingly, Lewis y antigen promoted the degradation of p27 by increasing ubiquitin-proteasome activity. In the vivo studies, Lewis y antigen improved the tumorigenic ability of ovarian cancer cells in nude mice and reduced the expression of p27. These findings suggested that Lewis y antigen activated both the autophagy and ubiquitin-proteasome activity and promoted the degradation of p27 through the ubiquitin-proteasome pathway.
Collapse
|
|
39
|
Hispolon suppresses metastasis via autophagic degradation of cathepsin S in cervical cancer cells. Cell Death Dis 2017; 8:e3089. [PMID: 28981104 PMCID: PMC5680581 DOI: 10.1038/cddis.2017.459] [Citation(s) in RCA: 71] [Impact Index Per Article: 8.9] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 02/06/2017] [Revised: 08/07/2017] [Accepted: 08/09/2017] [Indexed: 12/15/2022]
Abstract
Hispolon, a phenolic compound isolated from Phellinus igniarius, induces apoptosis and anti-tumor effects in cancers. However, the molecular mechanism involved in hispolon-mediated tumor-suppressing activities observed in cervical cancer is poorly characterized. Here, we demonstrated that treatment with hispolon inhibited cell metastasis in two cervical cancer cell lines. In addition, the downregulation of the lysosomal protease Cathepsin S (CTSS) was critical for hispolon-mediated suppression of tumor cell metastasis in both in vitro and in vivo models. Moreover, hispolon induced autophagy, which increased LC3 conversion and acidic vesicular organelle formation. Mechanistically, hispolon inhibited the cell motility of cervical cells through the extracellular signal-regulated kinase (ERK) pathway, and blocking of the ERK pathway reversed autophagy-mediated cell motility and CTSS inhibition. Our results indicate that autophagy is essential for decreasing CTSS activity to inhibit tumor metastasis by hispolon treatment in cervical cancer; this finding provides a new perspective on molecular regulation.
Collapse
|
|
40
|
Wang L, Li X, Yang Z, Pan X, Liu X, Zhu M, Xie J. Crotonaldehyde induces autophagy-mediated cytotoxicity in human bronchial epithelial cells via PI3K, AMPK and MAPK pathways. ENVIRONMENTAL POLLUTION (BARKING, ESSEX : 1987) 2017; 228:287-296. [PMID: 28551559 DOI: 10.1016/j.envpol.2017.03.083] [Citation(s) in RCA: 17] [Impact Index Per Article: 2.1] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Subscribe] [Scholar Register] [Received: 12/14/2016] [Revised: 03/25/2017] [Accepted: 03/29/2017] [Indexed: 06/07/2023]
Abstract
Crotonaldehyde is an ubiquitous hazardous pollutant in the environment which can be produced naturally, artificially and endogenously. Acute exposure of crotonaldehyde was reported to induce severe lung injury in humans and experimental animals. However, the exact toxicity mechanisms of crotonaldehyde in organisms have not been fully explored. In the present study, we explored the role autophagy played in the cytotoxicity induced by crotonaldehyde in human bronchial epithelial cells (BEAS-2B), and the pathways that mediated autophagy, including the phosphatidylinositol 3-kinase (PI3K) pathway, the AMP-activated protein kinase (AMPK) pathway and the mitogen-activated protein kinase (MAPK) pathways, were examined and validated. We found that crotonaldehyde induced cytotoxicity and autophagy simultaneously in BEAS-2B cells, and blockage of autophagic flux significantly elevated the viability of BEAS-2B exposed to high concentrations of crotonaldehyde. Crotonaldehyde down-regulated the activity of PI3K pathway, and elevated the activities of AMPK and MAPK pathways. Pretreatment of specific agonist or antagonist of these pathways could inhibit autophagy and partly improve the viability. These results suggested that acute exposure of crotonaldehyde induced cell death mediated by autophagy, which might be helpful to elucidate the toxicity mechanisms of crotonaldehyde and contribute to environmental and human health risk assessment.
Collapse
Affiliation(s)
- Limeng Wang
- Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023, PR China; University of Chinese Academy of Sciences, 19 Yuquan Road, Shijingshan District, Beijing 100049, PR China
| | - Xiang Li
- Key Laboratory of Tobacco Chemistry, Zhengzhou Tobacco Research Institute of CNTC, 2 Fengyang Road, Zhengzhou 450001, PR China
| | - Zhihua Yang
- Department of Radiation Toxicology and Oncology, Beijing Institute of Radiation Medicine, 27 Taiping Road, Beijing 100850, PR China
| | - Xiujie Pan
- Department of Radiation Toxicology and Oncology, Beijing Institute of Radiation Medicine, 27 Taiping Road, Beijing 100850, PR China
| | - Xingyu Liu
- Shanghai Tobacco Group Corporation of CNTC, 99 Wansheng South Street, Tongzhou District, Beijing 101121, PR China
| | - Maoxiang Zhu
- Department of Radiation Toxicology and Oncology, Beijing Institute of Radiation Medicine, 27 Taiping Road, Beijing 100850, PR China
| | - Jianping Xie
- Key Laboratory of Tobacco Chemistry, Zhengzhou Tobacco Research Institute of CNTC, 2 Fengyang Road, Zhengzhou 450001, PR China.
| |
Collapse
|
|
41
|
Seo BR, Min KJ, Woo SM, Choe M, Choi KS, Lee YK, Yoon G, Kwon TK. Inhibition of Cathepsin S Induces Mitochondrial ROS That Sensitizes TRAIL-Mediated Apoptosis Through p53-Mediated Downregulation of Bcl-2 and c-FLIP. Antioxid Redox Signal 2017; 27:215-233. [PMID: 27927016 DOI: 10.1089/ars.2016.6749] [Citation(s) in RCA: 36] [Impact Index Per Article: 4.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Indexed: 01/18/2023]
Abstract
AIMS Cathepsin S is highly expressed in various cancer cells, and it has protumoral effects, including promotion of migration, invasion, and neovascularization. In this study, we show that inhibition of cathepsin S could sensitize cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis. RESULTS An inhibitor of cathepsin S (Z-FL-COCHO; ZFL) markedly induced apoptosis in human renal cancer cells treated with TRAIL. In contrast, combined treatment with ZFL and TRAIL had no effect on normal cells. ZFL downregulated Bcl-2 expression at the transcriptional level in a p53-dependent manner, and overexpression of Bcl-2 also markedly blocked apoptosis induced by combined treatment with ZFL and TRAIL. In addition, ZFL induced downregulation of c-FLIP, and overexpression of c-FLIP blocked the apoptosis induced by ZFL plus TRAIL. Moreover, ZFL increased the expression of Cbl, an E3 ligase of c-FLIP, in a p53-dependent manner, and knockdown of Cbl markedly prevented c-FLIP downregulation and the apoptosis induced by ZFL plus TRAIL. Interestingly, ZFL induced p53 expression via production of mitochondrial reactive oxygen species (ROS). We also demonstrated that downregulation of cathepsin S by small interfering RNA sensitized TRAIL-mediated apoptosis in Caki cells. INNOVATION These results reveal the importance of cathepsin S on resistance against TRAIL, and inhibition of cathepsin S activity plays a crucial role in TRAIL-mediated cell death of cancer cells. CONCLUSION Our results indicated that inhibition of cathepsin S stimulates TRAIL-induced apoptosis through downregulation of Bcl-2 and Cbl-mediated c-FLIP by ROS-mediated p53 expression. Antioxid. Redox Signal. 27, 215-233.
Collapse
Affiliation(s)
- Bo Ram Seo
- 1 Department of Immunology, School of Medicine, Keimyung University , Daegu, South Korea
| | - Kyoung-Jin Min
- 1 Department of Immunology, School of Medicine, Keimyung University , Daegu, South Korea
| | - Seon Min Woo
- 1 Department of Immunology, School of Medicine, Keimyung University , Daegu, South Korea
| | - Misun Choe
- 2 Department of Pathology, School of Medicine, Keimyung University , Daegu, South Korea
| | - Kyeong Sook Choi
- 3 Department of Biochemistry & Molecular Biology, Ajou University School of Medicine , Suwon, South Korea
| | - Young-Kyung Lee
- 3 Department of Biochemistry & Molecular Biology, Ajou University School of Medicine , Suwon, South Korea
| | - Gyesoon Yoon
- 3 Department of Biochemistry & Molecular Biology, Ajou University School of Medicine , Suwon, South Korea
| | - Taeg Kyu Kwon
- 1 Department of Immunology, School of Medicine, Keimyung University , Daegu, South Korea
| |
Collapse
|
|
42
|
Zhang P, Zheng Z, Ling L, Yang X, Zhang N, Wang X, Hu M, Xia Y, Ma Y, Yang H, Wang Y, Liu H. w09, a novel autophagy enhancer, induces autophagy-dependent cell apoptosis via activation of the EGFR-mediated RAS-RAF1-MAP2K-MAPK1/3 pathway. Autophagy 2017; 13:1093-1112. [PMID: 28513279 DOI: 10.1080/15548627.2017.1319039] [Citation(s) in RCA: 49] [Impact Index Per Article: 6.1] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 01/09/2023] Open
Abstract
The EGFR (epidermal growth factor receptor) signaling pathway is frequently deregulated in many malignancies. Therefore, targeting the EGFR pathway is regarded as a promising strategy for anticancer drug discovery. Herein, we identified a 2-amino-nicotinonitrile compound (w09) as a novel autophagy enhancer, which potently induced macroautophagy/autophagy and consequent apoptosis in gastric cancer cells. Mechanistic studies revealed that EGFR-mediated activation of the RAS-RAF1-MAP2K-MAPK1/3 signaling pathway played a critical role in w09-induced autophagy and apoptosis of gastric cancer cells. Inhibition of the MAPK1/3 pathway with U0126 or blockade of autophagy by specific chemical inhibitors markedly attenuated the effect of w09-mediated growth inhibition and caspase-dependent apoptosis. Furthermore, these conclusions were supported by knockdown of ATG5 or knockout of ATG5 and/or ATG7. Notably, w09 increased the expression of SQSTM1 by transcription, and knockout of SQSTM1 or deleting the LC3-interaction region domain of SQSTM1, significantly inhibited w09-induced PARP1 cleavage, suggesting the central role played by SQSTM1 in w09-induced apoptosis. In addition, in vivo administration of w09 effectively inhibited tumor growth of SGC-7901 xenografts. Hence, our findings not only suggested that activation of the EGFR-RAS-RAF1-MAP2K-MAPK1/3 signaling pathway may play a critical role in w09-induced autophagy and apoptosis, but also imply that induction of autophagic cancer cell death through activation of the EGFR pathway may be a potential therapeutic strategy for EGFR-disregulated gastric tumors.
Collapse
Affiliation(s)
- Pinghu Zhang
- a Jiangsu Key Laboratory of New Drug Screening & Jiangsu Center for Pharmacodynamics Research and Evaluation & National Nanjing Center for Drug Screening & State Key Laboratory of Natural Medicines, China Pharmaceutical University , Nanjing , China
| | - Zuguo Zheng
- a Jiangsu Key Laboratory of New Drug Screening & Jiangsu Center for Pharmacodynamics Research and Evaluation & National Nanjing Center for Drug Screening & State Key Laboratory of Natural Medicines, China Pharmaceutical University , Nanjing , China
| | - Li Ling
- a Jiangsu Key Laboratory of New Drug Screening & Jiangsu Center for Pharmacodynamics Research and Evaluation & National Nanjing Center for Drug Screening & State Key Laboratory of Natural Medicines, China Pharmaceutical University , Nanjing , China
| | - Xiaohui Yang
- b Institute of Chemical Industry of Forestry Products, CAF, National Engineering Laboratory for Biomass Chemical Utilization & Key Laboratory of Forest Chemical Engineering, SFA & Key Lab. of Biomass Energy and Material , Nanjing , China
| | - Ni Zhang
- a Jiangsu Key Laboratory of New Drug Screening & Jiangsu Center for Pharmacodynamics Research and Evaluation & National Nanjing Center for Drug Screening & State Key Laboratory of Natural Medicines, China Pharmaceutical University , Nanjing , China
| | - Xue Wang
- a Jiangsu Key Laboratory of New Drug Screening & Jiangsu Center for Pharmacodynamics Research and Evaluation & National Nanjing Center for Drug Screening & State Key Laboratory of Natural Medicines, China Pharmaceutical University , Nanjing , China
| | | | - Yu Xia
- a Jiangsu Key Laboratory of New Drug Screening & Jiangsu Center for Pharmacodynamics Research and Evaluation & National Nanjing Center for Drug Screening & State Key Laboratory of Natural Medicines, China Pharmaceutical University , Nanjing , China
| | - Yiwen Ma
- a Jiangsu Key Laboratory of New Drug Screening & Jiangsu Center for Pharmacodynamics Research and Evaluation & National Nanjing Center for Drug Screening & State Key Laboratory of Natural Medicines, China Pharmaceutical University , Nanjing , China
| | - Haoran Yang
- a Jiangsu Key Laboratory of New Drug Screening & Jiangsu Center for Pharmacodynamics Research and Evaluation & National Nanjing Center for Drug Screening & State Key Laboratory of Natural Medicines, China Pharmaceutical University , Nanjing , China
| | - Yunyi Wang
- a Jiangsu Key Laboratory of New Drug Screening & Jiangsu Center for Pharmacodynamics Research and Evaluation & National Nanjing Center for Drug Screening & State Key Laboratory of Natural Medicines, China Pharmaceutical University , Nanjing , China
| | - Hongqi Liu
- d Infection and Immunity Laboratory, Institute of Medical Biology , Kunming National High-level Biosafety Primate Research Center, Chinese Academy of Medical Science & Peking Union of Medical School , Kunming , China
| |
Collapse
|
|
43
|
Inhibition of cathepsin S confers sensitivity to methyl protodioscin in oral cancer cells via activation of p38 MAPK/JNK signaling pathways. Sci Rep 2017; 7:45039. [PMID: 28327651 PMCID: PMC5361207 DOI: 10.1038/srep45039] [Citation(s) in RCA: 41] [Impact Index Per Article: 5.1] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/12/2016] [Accepted: 02/17/2017] [Indexed: 12/16/2022] Open
Abstract
Oral cancer is one of the most common cancers in the world. Approximately 90% of oral cancers are subtyped to oral squamous cell carcinoma (OSCC). Despite advances in diagnostic techniques and improvement in treatment modalities, the prognosis remains poor. Therefore, an effective chemotherapy mechanism that enhances tumor sensitivity to chemotherapeutics is urgently needed. Methyl protodioscin (MP) is a furostanol bisglycoside with a wide range of beneficial effects, including anti-inflammatory and anti-cancer properties. The aim of the present study was to determine the antitumor activity of MP on OSCC and its underlying mechanisms. Our results show that treatment of OSCC cells with MP potently inhibited cell viability. Moreover, MP leading to cell cycle arrest at G2/M phase, which subsequently activates caspase-3, -8, -9 and PARP to induce cell apoptosis. Meanwhile, we also demonstrate that MP induces a robust autophagy in OSCC cells. The results indicate cathepsin S (CTSS) is involved in MP-induced apoptosis and autophagy by modulation of p38 MAPK and JNK1/2 pathways. These findings may provide rationale to combine MP with CTSS blockade for the effective treatment of OSCC.
Collapse
|
|
44
|
Huang Y, Chu T, Liao T, Hu X, Huang B. Downregulation of lysosomal and further gene expression characterization in lung cancer patients with bone metastasis. ARTIFICIAL CELLS NANOMEDICINE AND BIOTECHNOLOGY 2016; 45:758-764. [PMID: 27683953 DOI: 10.1080/21691401.2016.1198364] [Citation(s) in RCA: 5] [Impact Index Per Article: 0.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Subscribe] [Scholar Register] [Indexed: 01/17/2023]
Abstract
Molecular and functional mechanisms of bone metastases were poorly understood. This study was to screen out differentially expressed genes (DEGs) and functional proteins in bone metastases from lung for better understanding of the molecular and functional mechanisms. Our results suggested CTSS, CTSD, MX1, NKX2-1 might play a decisive role in bone metastasis. Collectively, these results demonstrated that bone metastasis from lung cancer would lead to changes in lysosome function, which may affect the decomposition and elimination of old bone matrix, thus affecting bone turnover. In addition, our findings provided new insights into the prediction and treatment of bone metastases.
Collapse
Affiliation(s)
- Yong Huang
- a Department of Orthopedics , Third Military Medical University Xinqiao Hospital , Chong Qing , China
| | - Tongwei Chu
- a Department of Orthopedics , Third Military Medical University Xinqiao Hospital , Chong Qing , China
| | - Tongquan Liao
- a Department of Orthopedics , Third Military Medical University Xinqiao Hospital , Chong Qing , China
| | - Xu Hu
- a Department of Orthopedics , Third Military Medical University Xinqiao Hospital , Chong Qing , China
| | - Bo Huang
- a Department of Orthopedics , Third Military Medical University Xinqiao Hospital , Chong Qing , China
| |
Collapse
|
|
45
|
Ashley SL, Xia M, Murray S, O’Dwyer DN, Grant E, White ES, Flaherty KR, Martinez FJ, Moore BB. Six-SOMAmer Index Relating to Immune, Protease and Angiogenic Functions Predicts Progression in IPF. PLoS One 2016; 11:e0159878. [PMID: 27490795 PMCID: PMC4973878 DOI: 10.1371/journal.pone.0159878] [Citation(s) in RCA: 40] [Impact Index Per Article: 4.4] [Reference Citation Analysis] [Abstract] [MESH Headings] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/15/2016] [Accepted: 07/08/2016] [Indexed: 12/31/2022] Open
Abstract
RATIONALE Biomarkers in easily accessible compartments like peripheral blood that can predict disease progression in idiopathic pulmonary fibrosis (IPF) would be clinically useful regarding clinical trial participation or treatment decisions for patients. In this study, we used unbiased proteomics to identify relevant disease progression biomarkers in IPF. METHODS Plasma from IPF patients was measured using an 1129 analyte slow off-rate modified aptamer (SOMAmer) array, and patient outcomes were followed over the next 80 weeks. Receiver operating characteristic (ROC) curves evaluated sensitivity and specificity for levels of each biomarker and estimated area under the curve (AUC) when prognostic biomarker thresholds were used to predict disease progression. Both logistic and Cox regression models advised biomarker selection for a composite disease progression index; index biomarkers were weighted via expected progression-free days lost during follow-up with a biomarker on the unfavorable side of the threshold. RESULTS A six-analyte index, scaled 0 to 11, composed of markers of immune function, proteolysis and angiogenesis [high levels of ficolin-2 (FCN2), cathepsin-S (Cath-S), legumain (LGMN) and soluble vascular endothelial growth factor receptor 2 (VEGFsR2), but low levels of inducible T cell costimulator (ICOS) or trypsin 3 (TRY3)] predicted better progression-free survival in IPF with a ROC AUC of 0.91. An index score ≥ 3 (group ≥ 2) was strongly associated with IPF progression after adjustment for age, gender, smoking status, immunomodulation, forced vital capacity % predicted and diffusing capacity for carbon monoxide % predicted (HR 16.8, 95% CI 2.2-126.7, P = 0.006). CONCLUSION This index, derived from the largest proteomic analysis of IPF plasma samples to date, could be useful for clinical decision making in IPF, and the identified analytes suggest biological processes that may promote disease progression.
Collapse
Affiliation(s)
- Shanna L. Ashley
- Graduate Program in Immunology, University of Michigan, Ann Arbor, MI, United States of America
| | - Meng Xia
- Biostatistics Department, University of Michigan School of Public Health, Ann Arbor, MI, United States of America
| | - Susan Murray
- Biostatistics Department, University of Michigan School of Public Health, Ann Arbor, MI, United States of America
| | - David N. O’Dwyer
- Pulmonary and Critical Care Medicine Division, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, United States of America
| | - Ethan Grant
- MedImmune, Gaithersburg, MD, United States of America
| | - Eric S. White
- Pulmonary and Critical Care Medicine Division, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, United States of America
| | - Kevin R. Flaherty
- Pulmonary and Critical Care Medicine Division, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, United States of America
| | - Fernando J. Martinez
- Department of Internal Medicine, Weill Cornell Medical College, New York, NY, United States of America
| | - Bethany B. Moore
- Pulmonary and Critical Care Medicine Division, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, United States of America
- Department of Microbiology and Immunology, University of Michigan, Ann Arbor, MI, United States of America
| |
Collapse
|
|
46
|
Cathepsin S attenuates endosomal EGFR signalling: A mechanical rationale for the combination of cathepsin S and EGFR tyrosine kinase inhibitors. Sci Rep 2016; 6:29256. [PMID: 27387133 PMCID: PMC4937378 DOI: 10.1038/srep29256] [Citation(s) in RCA: 20] [Impact Index Per Article: 2.2] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 04/14/2016] [Accepted: 06/14/2016] [Indexed: 01/10/2023] Open
Abstract
EGF-mediated EGFR endocytosis plays a crucial role in the attenuation of EGFR activation by sorting from early endosomes to late endosomes and transporting them into lysosomes for the final proteolytic degradation. We previously observed that cathepsin S (CTSS) inhibition induces tumour cell autophagy through the EGFR-mediated signalling pathway. In this study, we further clarified the relationship between CTSS activities and EGFR signalling regulation. Our results revealed that CTSS can regulate EGFR signalling by facilitating EGF-mediated EGFR degradation. CTSS inhibition delayed the EGFR degradation process and caused EGFR accumulation in the late endosomes at the perinuclear region, which provides spatial compartments for prolonged EGFR and sustained downstream signal transducer and activator of transcription 3 and AKT signalling. Notably, cellular apoptosis was markedly enhanced by combining treatment with the EGFR inhibitor Iressa and CTSS inhibitor 6r. The data not only reveal a biological role of CTSS in EGFR signalling regulation but also evidence a rationale for its clinical evaluation in the combination of CTSS and EGFR tyrosine kinase inhibitors.
Collapse
|
|
47
|
Lonetti A, Cappellini A, Spartà AM, Chiarini F, Buontempo F, Evangelisti C, Evangelisti C, Orsini E, McCubrey JA, Martelli AM. PI3K pan-inhibition impairs more efficiently proliferation and survival of T-cell acute lymphoblastic leukemia cell lines when compared to isoform-selective PI3K inhibitors. Oncotarget 2016; 6:10399-414. [PMID: 25871383 PMCID: PMC4496363 DOI: 10.18632/oncotarget.3295] [Citation(s) in RCA: 30] [Impact Index Per Article: 3.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/14/2015] [Accepted: 02/06/2015] [Indexed: 11/25/2022] Open
Abstract
Class I phosphatidylinositol 3-kinases (PI3Ks) are frequently activated in T-cell acute lymphoblastic leukemia (T-ALL), mainly due to the loss of PTEN function. Therefore, targeting PI3Ks is a promising innovative approach for T-ALL treatment, however at present no definitive evidence indicated which is the better therapeutic strategy between pan or selective isoform inhibition, as all the four catalytic subunits might participate in leukemogenesis. Here, we demonstrated that in both PTEN deleted and PTEN non deleted T-ALL cell lines, PI3K pan-inhibition exerted the highest cytotoxic effects when compared to both selective isoform inhibition or dual p110γ/δ inhibition. Intriguingly, the dual p110γ/δ inhibitor IPI-145 was effective in Loucy cells, which are representative of early T-precursor (ETP)-ALL, a T-ALL subtype associated with a poor outcome. PTEN gene deletion did not confer a peculiar reliance of T-ALL cells on PI3K activity for their proliferation/survival, as PTEN was inactivated in PTEN non deleted cells, due to posttranslational mechanisms. PI3K pan-inhibition suppressed Akt activation and induced caspase-independent apoptosis. We further demonstrated that in some T-ALL cell lines, autophagy could exert a protective role against PI3K inhibition. Our findings strongly support clinical application of class I PI3K pan-inhibitors in T-ALL treatment, with the possible exception of ETP-ALL cases.
Collapse
Affiliation(s)
- Annalisa Lonetti
- Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy
| | - Alessandra Cappellini
- Department of Human, Social and Health Sciences, University of Cassino, Cassino, Italy
| | - Antonino Maria Spartà
- Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy
| | - Francesca Chiarini
- Muscoloskeletal Cell Biology Laboratory, IOR, Bologna, Italy.,Institute of Molecular Genetics, National Research Council-Rizzoli Orthopedic Institute, Bologna, Italy
| | - Francesca Buontempo
- Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy
| | - Camilla Evangelisti
- Muscoloskeletal Cell Biology Laboratory, IOR, Bologna, Italy.,Institute of Molecular Genetics, National Research Council-Rizzoli Orthopedic Institute, Bologna, Italy
| | - Cecilia Evangelisti
- Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy
| | - Ester Orsini
- Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy
| | - James A McCubrey
- Department of Microbiology and Immunology, Brody School of Medicine, East Carolina University, Greenville, NC, USA
| | | |
Collapse
|
|
48
|
Pawar K, Sharbati J, Einspanier R, Sharbati S. Mycobacterium bovis BCG Interferes with miR-3619-5p Control of Cathepsin S in the Process of Autophagy. Front Cell Infect Microbiol 2016; 6:27. [PMID: 27014637 PMCID: PMC4783571 DOI: 10.3389/fcimb.2016.00027] [Citation(s) in RCA: 21] [Impact Index Per Article: 2.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/09/2015] [Accepted: 02/22/2016] [Indexed: 12/14/2022] Open
Abstract
Main survival mechanism of pathogenic mycobacteria is to escape inimical phagolysosomal environment inside the macrophages. Many efforts have been made to unravel the molecular mechanisms behind this process. However, little is known about the involvement of microRNAs (miRNAs) in the regulation of phagolysosomal biosynthesis and maturation. Based on a bottom up approach, we searched for miRNAs that were involved in phagolysosomal processing events in the course of mycobacterial infection of macrophages. After infecting THP-1 derived macrophages with viable and heat killed Mycobacterium bovis BCG (BCG), early time points were identified after co-localization studies of the phagosomal marker protein LAMP1 and BCG. Differences in LAMP1 localization on the phagosomes of both groups were observed at 30 min and 4 h. After in silico based pre-selection of miRNAs, expression analysis at the identified time points revealed down-regulation of three miRNAs: miR-3619-5p, miR-637, and miR-324-3p. Consequently, most likely targets were predicted that were supposed to be mutually regulated by these three studied miRNAs. The lysosomal cysteine protease Cathepsin S (CTSS) and Rab11 family-interacting protein 4 (RAB11FIP4) were up-regulated and were considered to be connected to lysosomal trafficking and autophagy. Interaction studies verified the regulation of CTSS by miR-3619-5p. Down-regulation of CTSS by ectopic miR-3619-5p as well as its specific knockdown by siRNA affected the process of autophagy in THP-1 derived macrophages.
Collapse
Affiliation(s)
- Kamlesh Pawar
- Department of Veterinary Medicine, Institute of Veterinary Biochemistry, Freie Universität Berlin Berlin, Germany
| | - Jutta Sharbati
- Department of Veterinary Medicine, Institute of Veterinary Biochemistry, Freie Universität Berlin Berlin, Germany
| | - Ralf Einspanier
- Department of Veterinary Medicine, Institute of Veterinary Biochemistry, Freie Universität Berlin Berlin, Germany
| | - Soroush Sharbati
- Department of Veterinary Medicine, Institute of Veterinary Biochemistry, Freie Universität Berlin Berlin, Germany
| |
Collapse
|
|
49
|
Lysosomal cysteine peptidases – Molecules signaling tumor cell death and survival. Semin Cancer Biol 2015; 35:168-79. [DOI: 10.1016/j.semcancer.2015.08.001] [Citation(s) in RCA: 45] [Impact Index Per Article: 4.5] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/24/2015] [Revised: 07/31/2015] [Accepted: 08/03/2015] [Indexed: 12/18/2022]
|
|
50
|
Huang CC, Lee CC, Lin HH, Chen MC, Lin CC, Chang JY. Autophagy-Regulated ROS from Xanthine Oxidase Acts as an Early Effector for Triggering Late Mitochondria-Dependent Apoptosis in Cathepsin S-Targeted Tumor Cells. PLoS One 2015; 10:e0128045. [PMID: 26029922 PMCID: PMC4452096 DOI: 10.1371/journal.pone.0128045] [Citation(s) in RCA: 24] [Impact Index Per Article: 2.4] [Reference Citation Analysis] [Abstract] [MESH Headings] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/07/2014] [Accepted: 04/16/2015] [Indexed: 11/18/2022] Open
Abstract
Cathepsin S (CTSS), which is highly expressed in various malignant tumor cells, has been proposed to promote tumor progression, migration, and invasion. CTSS inhibition not only blocks tumor cell invasion and endothelial tube formation but also induces cellular cytotoxicity. In our previous studies, we have observed that CTSS inhibition induces autophagy, which is responsible for up-regulating xanthine oxidase for early ROS generation and consequent cell death. However, whether the autophagy-regulated early ROS triggers apoptosis remains unclear. We conducted a long-term follow-up study to investigate the relationship between early autophagy and late mitochondria-dependent apoptosis. We demonstrated that early ROS generation is critical for mitochondria damage and the activation of intrinsic apoptotic pathway. Attenuating the early ROS level diminished later mitochondrial damage and downstream apoptotic signaling. Collectively, mitochondria-dependent apoptosis is regulated by autophagy-regulated early ROS, which serves as an early effector that triggers mitochondrial signaling for late apoptosis. The data emphasize the essential role of autophagy-regulated early ROS in triggering late apoptotic signaling.
Collapse
Affiliation(s)
- Chien-Chang Huang
- National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan, ROC
| | - Cheng-Che Lee
- National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan, ROC
| | - Hsiao-Han Lin
- National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan, ROC
- The Institute of Basic Medical Sciences, College of Medicine, National Cheng Kung University, Tainan, Taiwan, ROC
| | - Mei-Chi Chen
- National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan, ROC
| | - Chun-Cheng Lin
- Department of Chemistry, National Tsing Hua University, Hsinchu, Taiwan, ROC
| | - Jang-Yang Chang
- National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan, ROC
- Division of Hematology and Oncology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan, ROC
- Institute of Molecular Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan, ROC
- * E-mail:
| |
Collapse
|