|
1
|
Feng Y, Li X, Sun F, Zhou J, Wang L, Zeng H, Yu J. Efficacy and Safety Analysis of Recombinant Human Endostatin (Endostar) Combined With Chemoradiotherapy for Locally Advanced Cervical Cancer: A 2-Center Retrospective Study. Technol Cancer Res Treat 2024; 23:15330338241263026. [PMID: 39043041 PMCID: PMC11271135 DOI: 10.1177/15330338241263026] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/05/2023] [Revised: 05/08/2024] [Accepted: 05/24/2024] [Indexed: 07/25/2024] Open
Abstract
BACKGROUND This study aims to assess the efficacy and safety of Endostar in the management of locally advanced cervical cancer. METHODS This retrospective, 2-center study enrolled 41 patients with locally advanced cervical cancer between June 2017 and December 2020. The patients were subjected to a combination of Endostar and chemoradiotherapy until they experienced disease progression or an unacceptable level of toxicity. The patients in the Endostar combined chemoradiotherapy (E + CRT) and CRT groups were matched 1:1 based on clinical features, including age, disease stage, and pathological type. The therapeutic efficacy and safety outcomes were compared between the 2 groups. RESULTS Early treatment response: the CR rates in E + CRT and CRT groups were 48.8% and 26.8%, respectively (χ2 = 4.20, P < .05). The ORR and DCR were not significantly different between the 2 groups. Long-term efficacy: there was no significant difference in the 1-year and 2-year PFS rates and OS rates between 2 groups. However, in patients with stage IIB, subgroup analyses revealed a significant difference in PFS between the 2 groups (P < .05). Prognostic factors: stage, Eastern Cooperative Oncology Group (ECOG) score, and tumor size were independent predictive factors for PFS, while ECOG score and tumor size were those of OS in patients with locally advanced cervical cancer. Safety: The incidence of grade III-IV myelosuppression was significantly lower in E + CRT group than in CRT group (P < .05). CONCLUSIONS The combination of Endostar and concurrent CRT exhibited greater efficacy in treating locally advanced cervical cancer with no severe adverse reactions, when compared to simple CRT. It is expected that this approach will evolve into a new treatment alternative for patients with locally advanced cervical cancer.
Collapse
Affiliation(s)
- Yue Feng
- Department of Radiotherapy, ShuGuang Hospital, The Affiliated Hospital of Shanghai University of Chinese Traditional Medicine, Shanghai, China
| | - Xin Li
- Department of Radiotherapy, Changzhou No.2 People's Hospital, the Affiliated Hospital of Nanjing Medical University, Changzhou, Jiangsu Province, China
| | - Fei Sun
- Department of Radiotherapy, Changzhou No.2 People's Hospital, the Affiliated Hospital of Nanjing Medical University, Changzhou, Jiangsu Province, China
| | - Juying Zhou
- Department of Radiotherapy, The First People's Hospital of Soochow University, Suzhou, Jiangsu Province, China
| | - Lili Wang
- Department of Radiotherapy, The First People's Hospital of Soochow University, Suzhou, Jiangsu Province, China
| | - Hongwei Zeng
- Department of Radiotherapy, ShuGuang Hospital, The Affiliated Hospital of Shanghai University of Chinese Traditional Medicine, Shanghai, China
| | - Jingping Yu
- Department of Radiotherapy, ShuGuang Hospital, The Affiliated Hospital of Shanghai University of Chinese Traditional Medicine, Shanghai, China
| |
Collapse
|
|
2
|
Jiang S, Zhou Y, Zou L, Chu L, Chu X, Ni J, Li Y, Guo T, Yang X, Zhu Z. Low- dose Apatinib promotes vascular normalization and hypoxia reduction and sensitizes radiotherapy in lung cancer. Cancer Med 2023; 12:4434-4445. [PMID: 36065943 PMCID: PMC9972072 DOI: 10.1002/cam4.5113] [Citation(s) in RCA: 3] [Impact Index Per Article: 3.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 05/16/2022] [Revised: 07/08/2022] [Accepted: 07/11/2022] [Indexed: 11/10/2022] Open
Abstract
BACKGROUND AND PURPOSE Abnormal vascular network of tumor can create a hypoxic microenvironment, and reduce radiotherapy sensitivity. Normalization of tumor vasculature can be a new therapeutic strategy for sensitizing radiotherapy. This study aimed to explore the effect of apatinib on vascular normalization, as well as the syngeneic effect with radiotherapy on lung cancer. MATERIALS AND METHODS Lewis lung carcinoma (LLC) xenograft-bearing female C57BL/6 mice were treated with different doses of apatinib (30, 60, and 120 mg/kg per day) and/or radiation therapy (8 Gy/1F) and then sacrificed to harvest tumor tissue for immunohistochemical test. Further 18 F-FMISO micro- PET in vivo explored the degree of hypoxia. RESULTS Immunohistochemistry of CD31 and alpha-smooth muscle actin (α-SMA) proved that low-dose apatinib can normalize vasculature in tumor, especially on Day 10. Tissue staining of hypoxyprobe-1 and 18 F-FMISO micro- PET in vivo showed that 60 mg/kg/day of apatinib significantly alleviates hypoxia. Moreover, this study further proved that low-dose apatinib (60 mg/kg/day) can enhance the radio-response of LLC xenograft mice. CONCLUSION Our data suggested that low- dose apatinib can successfully induce a vascular normalization window and function as a radio- sensitizer in the lung cancer xenografts model.
Collapse
Affiliation(s)
- Shanshan Jiang
- Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
| | - Yue Zhou
- Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
| | - Liqing Zou
- Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
| | - Li Chu
- Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
| | - Xiao Chu
- Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
| | - Jianjiao Ni
- Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
| | - Yida Li
- Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
| | - Tiantian Guo
- Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
| | - Xi Yang
- Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
| | - Zhengfei Zhu
- Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.,Institute of Thoracic Oncology, Fudan University, Shanghai, China
| |
Collapse
|
|
3
|
Cunningham C, Bolcaen J, Bisio A, Genis A, Strijdom H, Vandevoorde C. Recombinant Endostatin as a Potential Radiosensitizer in the Treatment of Non-Small Cell Lung Cancer. Pharmaceuticals (Basel) 2023; 16:219. [PMID: 37259367 PMCID: PMC9961924 DOI: 10.3390/ph16020219] [Citation(s) in RCA: 1] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/20/2022] [Revised: 01/22/2023] [Accepted: 01/24/2023] [Indexed: 11/03/2023] Open
Abstract
Non-small cell lung cancer (NSCLC) is the most prevalent type of lung cancer, which is the leading cause of cancer-related deaths worldwide. Over the past decades, tumour angiogenesis has been intensely studied in the treatment of NSCLC due to its fundamental role in cancer progression. Several anti-angiogenic drugs, such as recombinant endostatin (RE), have been evaluated in several preclinical and clinical trials, with mixed and often disappointing results. However, there is currently an emerging interest in RE due to its ability to create a vascular normalization window, which could further improve treatment efficacy of the standard NSCLC treatment. This review provides an overview of preclinical and clinical studies that combined RE and radiotherapy for NSCLC treatment. Furthermore, it highlights the ongoing challenges that have to be overcome in order to maximize the benefit; as well as the potential advantage of combinations with particle therapy and immunotherapy, which are rapidly gaining momentum in the treatment landscape of NSCLC. Different angiogenic and immunosuppressive effects are observed between particle therapy and conventional X-ray radiotherapy. The combination of RE, particle therapy and immunotherapy presents a promising future therapeutic triad for NSCLC.
Collapse
Affiliation(s)
- Charnay Cunningham
- Centre for Cardio-Metabolic Research in Africa (CARMA), Division of Medical Physiology, Stellenbosch University, Cape Town 7602, South Africa
- Radiation Biophysics Division, SSC Laboratory, NRF Ithemba LABS, Cape Town 7131, South Africa
| | - Julie Bolcaen
- Radiation Biophysics Division, SSC Laboratory, NRF Ithemba LABS, Cape Town 7131, South Africa
| | - Alessandra Bisio
- Department of Cellular, Computational and Integrative Biology—CIBIO, University of Trento, 38123 Trento, Italy
| | - Amanda Genis
- Centre for Cardio-Metabolic Research in Africa (CARMA), Division of Medical Physiology, Stellenbosch University, Cape Town 7602, South Africa
| | - Hans Strijdom
- Centre for Cardio-Metabolic Research in Africa (CARMA), Division of Medical Physiology, Stellenbosch University, Cape Town 7602, South Africa
| | - Charlot Vandevoorde
- Biophysics Department, GSI Helmholtzzentrum für Schwerionenforschung, Planckstr. 1, 64291 Darmstadt, Germany
| |
Collapse
|
|
4
|
Wang XY, Beeraka NM, Xue NN, Yu HM, Yang Y, Liu MX, Nikolenko VN, Liu JQ, Zhao D. Identification of a three-gene prognostic signature for radioresistant esophageal squamous cell carcinoma. World J Clin Oncol 2023; 14:13-26. [PMID: 36699628 PMCID: PMC9850665 DOI: 10.5306/wjco.v14.i1.13] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 07/25/2022] [Revised: 10/25/2022] [Accepted: 12/06/2022] [Indexed: 01/10/2023] Open
Abstract
BACKGROUND Esophageal squamous cell carcinoma (ESCC) is causing a high mortality rate due to the lack of efficient early prognosis markers and suitable therapeutic regimens. The prognostic role of genes responsible for the acquisition of radioresistance in ESCC has not been fully elucidated.
AIM To establish a prognostic model by studying gene expression patterns pertinent to radioresistance in ESCC patients.
METHODS Datasets were obtained from the Gene Expression Omnibus and The Cancer Genome Atlas databases. The edgeR, a Bioconductor package, was used to analyze mRNA expression between different groups. We screened genes specifically responsible for radioresistance to estimate overall survival. Pearson correlation analysis was performed to confirm whether the expression of those genes correlated with each other. Genes contributing to radioresistance and overall survival were assessed by the multivariate Cox regression model through the calculation of βi and risk score using the following formula: 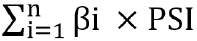 . .
RESULTS We identified three prognostic mRNAs (cathepsin S [CTSS], cluster of differentiation 180 [CD180], and SLP adapter and CSK-interacting membrane protein [SCIMP]) indicative of radioresistance. The expression of the three identified mRNAs was related to each other (r > 0.70 and P < 0.05). As to 1-year and 3-year overall survival prediction, the area under the time-dependent receiver operating characteristic curve of the signature consisting of the three mRNAs was 0.716 and 0.841, respectively. When stratifying patients based on the risk score derived from the signature, the high-risk group exhibited a higher death risk and shorter survival time than the low-risk group (P < 0.0001). Overall survival of the low-risk patients was significantly better than that of the high-risk patients (P = 0.018).
CONCLUSION We have developed a novel three-gene prognostic signature consisting of CTSS, CD180, and SCIMO for ESCC, which may facilitate the prediction of early prognosis of this malignancy.
Collapse
Affiliation(s)
- Xiao-Yan Wang
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Narasimha M Beeraka
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- Department of Pharmaceutical Chemistry, JSS College of Pharmacy, Mysuru 570015, India
| | - Nan-Nan Xue
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Hui-Ming Yu
- Department of Radiation Oncology, Peking University Cancer Hospital & Institute, Beijing 065005, China
| | - Ya Yang
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Mao-Xing Liu
- Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Gastrointestinal Surgery IV, Peking University Cancer Hospital & Institute, Beijing, China
| | - Vladimir N Nikolenko
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- M.V. Lomonosov Moscow State University, Moscow 119991, Russia
| | - Jun-Qi Liu
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Di Zhao
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| |
Collapse
|
|
5
|
Sha Y, Hong H, Cai W, Sun T. Single-Cell Transcriptomics of Endothelial Cells in Upper and Lower Human Esophageal Squamous Cell Carcinoma. Curr Oncol 2022; 29:7680-7694. [PMID: 36290884 PMCID: PMC9600084 DOI: 10.3390/curroncol29100607] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/14/2022] [Revised: 09/22/2022] [Accepted: 10/07/2022] [Indexed: 11/26/2022] Open
Abstract
Esophageal squamous cell carcinoma (ESCC) is a type of progressive and distant metastatic tumor. Targeting anti-angiogenic genes could effectively hinder ESCC development and metastasis, whereas ESCC locating on the upper or the lower esophagus showed different response to the same clinical treatment, suggesting ESCC location should be taken into account when exploring new therapeutic targets. In the current study, to find novel anti-angiogenic therapeutic targets, we identified endothelial cell subsets in upper and lower human ESCC using single-cell RNA sequencing (scRNA-seq), screened differentially expressed genes (DEGs), and performed gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. The results showed that common DEGs shared in the upper and the lower endothelial cells mainly are involved in vessel development, angiogenesis, and cell motility of endothelial cells by regulating PI3K-AKT, Rap1, Ras, TGF-beta, and Apelin signaling pathways. The critical regulatory genes were identified as ITGB1, Col4A1, Col4A2, ITGA6, LAMA4, LAMB1, LAMC1, VWF, ITGA5, THBS1, PDGFB, PGF, RHOC, and CTNNB1. Cell metabolism-relevant genes, e.g., MGST3, PNP, UPP1, and HYAL2 might be the prospective therapeutic targets. Furthermore, we found that DEGs only in the upper endothelial cells, such as MAPK3, STAT3, RHOA, MAPK11, HIF1A, FGFR1, GNG5, GNB1, and ARHGEF12, mainly regulated cell adhesion, structure morphogenesis, and motility through Phospholipase D, Apelin, and VEGF signaling pathways. Moreover, DEGs only in the lower endothelial cells, for instance PLCG2, EFNA1, CALM1, and RALA, mainly regulated cell apoptosis and survival by targeting calcium ion transport through Rap1, Ras, cAMP, Phospholipase D, and Phosphatidylinositol signaling pathways. In addition, the upper endothelial cells showed significant functional diversity such as cytokine-responsive, migratory, and proliferative capacity, presenting a better angiogenic capacity and making it more sensitive to anti-angiogenic therapy compared with the lower endothelial cells. Our study has identified the potential targeted genes for anti-angiogenic therapy for both upper and lower ESCC, and further indicated that anti-angiogenic therapy might be more effective for upper ESCC, which still need to be further examined in the future.
Collapse
Affiliation(s)
- Yongqiang Sha
- Center for Precision Medicine, School of Medicine and School of Biomedical Sciences, Huaqiao University, Xiamen 361021, China
| | - Huhai Hong
- Center for Precision Medicine, School of Medicine and School of Biomedical Sciences, Huaqiao University, Xiamen 361021, China
| | - Wenjie Cai
- Departments of Radiation Oncology, First Hospital of Quanzhou Affiliated to Fujian Medical University, Quanzhou 362000, China
- Correspondence: (W.C.); (T.S.)
| | - Tao Sun
- Center for Precision Medicine, School of Medicine and School of Biomedical Sciences, Huaqiao University, Xiamen 361021, China
- Correspondence: (W.C.); (T.S.)
| |
Collapse
|
|
6
|
Li X, Su X, Yan C, Ma Y, Li H, Xia J, Li H, Jiang Q, Zhou L, Zou Z. Role of vascular endothelial growth factor in radiotherapy resistance to esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 2022; 149:2543-2550. [PMID: 35767192 DOI: 10.1007/s00432-022-04122-x] [Citation(s) in RCA: 1] [Impact Index Per Article: 0.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 05/13/2022] [Accepted: 06/07/2022] [Indexed: 10/17/2022]
Abstract
Vascular endothelial growth factor (VEGF) is related to the radiation resistance of tumors, resulting in the failure of tumor radiotherapy. The purpose of this study was to discuss the role of VEGF in radiotherapy resistance of esophageal squamous cell carcinoma (ESCC). We used the VEGF kit by ELISA to detect the serum VEGF level of ESCC patients who only received radiotherapy. The expression of VEGF in ESCC cells after siRNA treatment was verified by Western blot. The sensitivity of ESCC cells to radiation after knocking down VEGF was analyzed by Clonogenic assay and Cell counting kit (CCK-8). The results showed that the level of serum VEGF in patients with ESCC before and after radiotherapy was related to the clinical response, and it was confirmed that knocking down the expression of VEGF in ESCC cells improved the sensitivity to radiation.
Collapse
Affiliation(s)
- Xin Li
- Department of Radiation Oncology, Huai'an Hospital Affiliated to Xuzhou Medical University, Huai'an City, Jiangsu Province, China
| | - Xinyu Su
- Department of Radiation Oncology, The Comprehensive Cancer Centre of Nanjing Drum Tower Hospital, Affiliated Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China
| | - Chen Yan
- Department of Radiation Oncology, Huai'an Hospital Affiliated to Xuzhou Medical University, Huai'an City, Jiangsu Province, China
| | - Yuanyuan Ma
- Department of Radiation Oncology, Huai'an Hospital Affiliated to Xuzhou Medical University, Huai'an City, Jiangsu Province, China
| | - Heng Li
- Department of Radiation Oncology, Huai'an Hospital Affiliated to Xuzhou Medical University, Huai'an City, Jiangsu Province, China
| | - Jianhong Xia
- Department of Radiation Oncology, Huai'an Hospital Affiliated to Xuzhou Medical University, Huai'an City, Jiangsu Province, China
| | - Hongliang Li
- Department of Radiation Oncology, Huai'an Hospital Affiliated to Xuzhou Medical University, Huai'an City, Jiangsu Province, China
| | - Qian Jiang
- Department of Radiation Oncology, Huai'an Hospital Affiliated to Xuzhou Medical University, Huai'an City, Jiangsu Province, China
| | - Liqing Zhou
- Department of Radiation Oncology, Huai'an Hospital Affiliated to Xuzhou Medical University, Huai'an City, Jiangsu Province, China.
| | - Zhengyun Zou
- Department of Radiation Oncology, The Comprehensive Cancer Centre of Nanjing Drum Tower Hospital, Affiliated Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China. .,Department of Radiation Oncology, The Comprehensive Cancer Centre of Nanjing Drum Tower Hospital, Clinical College of Nanjing Medical University, Nanjing, China. .,Department of Radiation Oncology, The Comprehensive Cancer Centre of Nanjing Drum Tower Hospital, Clinical College of Nanjing University of Chinese Medicine, Nanjing, China.
| |
Collapse
|
|
7
|
Wang N, Gao Q, Tang J, Jiang Y, Yang L, Shi X, Chen Y, Zhang Y, Fu S, Lin S. Anti-tumor effect of local injectable hydrogel-loaded endostatin alone and in combination with radiotherapy for lung cancer. Drug Deliv 2021; 28:183-194. [PMID: 33427520 PMCID: PMC7808389 DOI: 10.1080/10717544.2020.1869864] [Citation(s) in RCA: 4] [Impact Index Per Article: 1.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 01/30/2023] Open
Abstract
Endostatin (ES) can effectively inhibit neovascularization in most solid tumors and has the potential to make oxygen delivery more efficient and increase the efficacy of radiotherapy (RT). With a short half-life, ES is mainly administered systemically, which leads to low intake in tumor tissue and often toxic systemic side effects. In this study, we used hyaluronic acid-tyramine as a carrier to synthesize an ES-loaded hydrogel drug (ES/HA-Tyr) that can be injected locally. ES/HA-Tyr has a longer half-life and fewer systemic toxic side effects, and it exerts a better anti-angiogenic effect and anti-tumor effect with RT. In vitro, ES/HA-Tyr showed sustained release in the release assay and a stronger ability to inhibit the proliferation of human umbilical vascular endothelial cells (HUVECs) in the MTT assay; it exhibited a more potent effect against HUVEC invasion and a stronger anti-angiogenic effect on HUVECs in tube formation. In vivo, ES/HA-Tyr increased local drug concentration, decreased blood drug concentration, and caused less systemic toxicity. Further, ES/HA-Tyr effectively reduced tumor microvessel density, increased tumor pericyte coverage, decreased tumor hypoxia, and increased RT response. ES/HA-Tyr + RT also had increased anti-tumor and anti-angiogenic effects in Lewis lung cancer (LLC) xenograft models. In conclusion, ES/HA-Tyr showed sustained release, lower systemic toxicity, and better anti-tumor effects than ES. In addition, ES/HA-Tyr + RT enhanced anti-angiogenic effects, reduced tumor hypoxia, and increased the efficacy of RT in LLC-bearing mice.
Collapse
Affiliation(s)
- Na Wang
- Department of Oncology, Affiliated Hospital of Southwest Medical University, Luzhou, China.,Department of Oncology, Zigong First People's Hospital, Zigong, China
| | - Qin Gao
- Department of Oncology, Affiliated Hospital of Southwest Medical University, Luzhou, China
| | - Juan Tang
- Department of Oncology, Affiliated Hospital of Southwest Medical University, Luzhou, China
| | - YiQing Jiang
- Department of Oncology, Affiliated Hospital of Southwest Medical University, Luzhou, China
| | - LiShi Yang
- Department of Oncology, Affiliated Hospital of Southwest Medical University, Luzhou, China
| | - XiangXiang Shi
- Department of Oncology, Affiliated Hospital of Southwest Medical University, Luzhou, China
| | - Yue Chen
- Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province, Affiliated Hospital of Southwest Medical University, Luzhou, China
| | - Yan Zhang
- Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province, Affiliated Hospital of Southwest Medical University, Luzhou, China
| | - ShaoZhi Fu
- Department of Oncology, Affiliated Hospital of Southwest Medical University, Luzhou, China
| | - Sheng Lin
- Department of Oncology, Affiliated Hospital of Southwest Medical University, Luzhou, China
| |
Collapse
|
|
8
|
Davern M, Donlon NE, Power R, Hayes C, King R, Dunne MR, Reynolds JV. The tumour immune microenvironment in oesophageal cancer. Br J Cancer 2021; 125:479-494. [PMID: 33903730 PMCID: PMC8368180 DOI: 10.1038/s41416-021-01331-y] [Citation(s) in RCA: 18] [Impact Index Per Article: 6.0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/26/2020] [Revised: 01/16/2021] [Accepted: 02/17/2021] [Indexed: 02/02/2023] Open
Abstract
Oesophageal cancer (OC) is an inflammation-associated malignancy linked to gastro-oesophageal reflux disease, obesity and tobacco use. Knowledge of the microenvironment of oesophageal tumours is relevant to our understanding of the development of OC and its biology, and has major implications for understanding the response to standard therapies and immunotherapies, as well as for uncovering novel targets. In this context, we discuss what is known about the TME in OC from tumour initiation to development and progression, and how this is relevant to therapy sensitivity and resistance in the two major types of OC. We provide an immunological characterisation of the OC TME and discuss its prognostic implications with specific comparison with the Immunoscore and immune-hot, -cold, altered-immunosuppressed and -altered-excluded models. Targeted therapeutics for the TME under pre-clinical and clinical investigation in OCs are also summarised. A deeper understanding of the TME will enable the development of combination approaches to concurrently target the tumour cells and TME delivering precision medicine to OC patients.
Collapse
Affiliation(s)
- Maria Davern
- Department of Surgery, School of Medicine, Trinity College Dublin, Dublin, Ireland
- Trinity St James's Cancer Institute, St James's Hospital, Dublin, Ireland
| | - Noel E Donlon
- Department of Surgery, School of Medicine, Trinity College Dublin, Dublin, Ireland
- Trinity St James's Cancer Institute, St James's Hospital, Dublin, Ireland
| | - Robert Power
- Department of Surgery, School of Medicine, Trinity College Dublin, Dublin, Ireland
- Trinity St James's Cancer Institute, St James's Hospital, Dublin, Ireland
| | - Conall Hayes
- Department of Surgery, School of Medicine, Trinity College Dublin, Dublin, Ireland
- Trinity St James's Cancer Institute, St James's Hospital, Dublin, Ireland
| | - Ross King
- Department of Surgery, School of Medicine, Trinity College Dublin, Dublin, Ireland
- Trinity St James's Cancer Institute, St James's Hospital, Dublin, Ireland
| | - Margaret R Dunne
- Department of Surgery, School of Medicine, Trinity College Dublin, Dublin, Ireland
- Trinity St James's Cancer Institute, St James's Hospital, Dublin, Ireland
| | - John V Reynolds
- Department of Surgery, School of Medicine, Trinity College Dublin, Dublin, Ireland.
- Trinity St James's Cancer Institute, St James's Hospital, Dublin, Ireland.
| |
Collapse
|
|
9
|
ECM Remodeling in Squamous Cell Carcinoma of the Aerodigestive Tract: Pathways for Cancer Dissemination and Emerging Biomarkers. Cancers (Basel) 2021. [DOI: 10.3390/cancers13112759
expr 955442319 + 839973387] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 03/16/2023] Open
Abstract
Squamous cell carcinomas (SCC) include a number of different types of tumors developing in the skin, in hollow organs, as well as the upper aerodigestive tract (UADT) including the head and neck region and the esophagus which will be dealt with in this review. These tumors are often refractory to current therapeutic approaches with poor patient outcome. The most important prognostic determinant of SCC tumors is the presence of distant metastasis, significantly correlating with low patient survival rates. Rapidly emerging evidence indicate that the extracellular matrix (ECM) composition and remodeling profoundly affect SSC metastatic dissemination. In this review, we will summarize the current knowledge on the role of ECM and its remodeling enzymes in affecting the growth and dissemination of UADT SCC. Taken together, these published evidence suggest that a thorough analysis of the ECM composition in the UADT SCC microenvironment may help disclosing the mechanism of resistance to the treatments and help defining possible targets for clinical intervention.
Collapse
|
|
10
|
ECM Remodeling in Squamous Cell Carcinoma of the Aerodigestive Tract: Pathways for Cancer Dissemination and Emerging Biomarkers. Cancers (Basel) 2021; 13:cancers13112759. [PMID: 34199373 PMCID: PMC8199582 DOI: 10.3390/cancers13112759] [Citation(s) in RCA: 6] [Impact Index Per Article: 2.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/31/2021] [Revised: 05/27/2021] [Accepted: 05/28/2021] [Indexed: 12/12/2022] Open
Abstract
Simple Summary Local and distant metastasis of patients affected by squamous cell carcinoma of the upper aerodigestive tract predicts poor prognosis. In the latest years, the introduction of new therapeutic approaches, including targeted and immune therapies, has improved the overall survival. However, a large number of these patients do not benefit from these treatments. Thus, the identification of suitable prognostic and predictive biomarkers, as well as the discovery of new therapeutic targets have emerged as a crucial clinical need. In this context, the extracellular matrix represents a suitable target for the development of such therapeutic tools. In fact, the extracellular matrix is composed by complex molecules able to interact with a plethora of receptors and growth factors, thus modulating the dynamic crosstalk between cancer cells and the tumor microenvironment. In this review, we summarize the current knowledge of the role of the extracellular matrix in affecting squamous cell carcinoma growth and dissemination. Despite extracellular matrix is known to affect the development of many cancer types, only a restricted number of these molecules have been recognized to impact on squamous cell carcinoma progression. Thus, we consider that a thorough analysis of these molecules may be key to develop new potential therapeutic targets/biomarkers. Abstract Squamous cell carcinomas (SCC) include a number of different types of tumors developing in the skin, in hollow organs, as well as the upper aerodigestive tract (UADT) including the head and neck region and the esophagus which will be dealt with in this review. These tumors are often refractory to current therapeutic approaches with poor patient outcome. The most important prognostic determinant of SCC tumors is the presence of distant metastasis, significantly correlating with low patient survival rates. Rapidly emerging evidence indicate that the extracellular matrix (ECM) composition and remodeling profoundly affect SSC metastatic dissemination. In this review, we will summarize the current knowledge on the role of ECM and its remodeling enzymes in affecting the growth and dissemination of UADT SCC. Taken together, these published evidence suggest that a thorough analysis of the ECM composition in the UADT SCC microenvironment may help disclosing the mechanism of resistance to the treatments and help defining possible targets for clinical intervention.
Collapse
|
|
11
|
Liu Z, Zhao Q, Zheng Z, Liu S, Meng L, Dong L, Jiang X. Vascular normalization in immunotherapy: A promising mechanisms combined with radiotherapy. Biomed Pharmacother 2021; 139:111607. [PMID: 33965730 DOI: 10.1016/j.biopha.2021.111607] [Citation(s) in RCA: 24] [Impact Index Per Article: 8.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/26/2021] [Revised: 04/02/2021] [Accepted: 04/12/2021] [Indexed: 02/07/2023] Open
Abstract
Leakage and compression of blood vessels may result in deprivation of blood flow to a large number of tumor tissues, which can lead to tumor hypoxia. Hypoxia induces an increase in the expression of hypoxia-inducible factor 1 in tumor cells, which induces angiogenesis in tumors through the high expression of vascular endothelial growth factor, thereby forming a positive feedback vicious circle. Improving hypoxia by normalizing blood vessels and improving radiosensitivity by immunotherapy has emerged as a new application of combined immunotherapy and radiotherapy. Interferon γ produced by CD4 + /CD8 + T cells, induced by immune checkpoint inhibitors, plays an important role in the normalization of blood vessels; tumor-associated eosinophils also play a role in the process of immunotherapy-induced blood vessel normalization. In addition, the reduction in regulatory T cells induced by immune checkpoint inhibitors can increase eosinophil levels, which promotes the further development of vascular normalization mechanisms. This review focuses on the mechanism of immunotherapy to normalize blood vessels, and proposes a good prospect for improving hypoxia. Due to the narrow vascular normalization window of anti-angiogenesis therapy, discovery of the vascular normalization effect of immunotherapy provides a new idea for the combined application of immunotherapy and radiotherapy. The enlarged vascular normalization window and improved hypoxia provide a good opportunity for the subsequent implementation of radiotherapy. The above sorting and analysis may pave the way for a promising strategy for cancer treatment via combined immunotherapy and radiotherapy.
Collapse
Affiliation(s)
- Zijing Liu
- Department of Radiation Oncology, The First Hospital of Jilin University, Changchun 130021, China; Jilin Provincial Key Laboratory of Radiation Oncology & Therapy, The First Hospital of Jilin University, Changchun 130021, China; NHC Key Laboratory of Radiobiology, School of Public Health, Jilin University, Changchun 130021, China
| | - Qin Zhao
- Department of Radiation Oncology, The First Hospital of Jilin University, Changchun 130021, China; Jilin Provincial Key Laboratory of Radiation Oncology & Therapy, The First Hospital of Jilin University, Changchun 130021, China; NHC Key Laboratory of Radiobiology, School of Public Health, Jilin University, Changchun 130021, China
| | - Zhuangzhuang Zheng
- Department of Radiation Oncology, The First Hospital of Jilin University, Changchun 130021, China; Jilin Provincial Key Laboratory of Radiation Oncology & Therapy, The First Hospital of Jilin University, Changchun 130021, China; NHC Key Laboratory of Radiobiology, School of Public Health, Jilin University, Changchun 130021, China
| | - Shiyu Liu
- Department of Radiation Oncology, The First Hospital of Jilin University, Changchun 130021, China; Jilin Provincial Key Laboratory of Radiation Oncology & Therapy, The First Hospital of Jilin University, Changchun 130021, China; NHC Key Laboratory of Radiobiology, School of Public Health, Jilin University, Changchun 130021, China
| | - Lingbin Meng
- Department of Hematology and Medical Oncology, Moffitt Cancer Center, Tampa, FL 33612, USA
| | - Lihua Dong
- Department of Radiation Oncology, The First Hospital of Jilin University, Changchun 130021, China; Jilin Provincial Key Laboratory of Radiation Oncology & Therapy, The First Hospital of Jilin University, Changchun 130021, China; NHC Key Laboratory of Radiobiology, School of Public Health, Jilin University, Changchun 130021, China.
| | - Xin Jiang
- Department of Radiation Oncology, The First Hospital of Jilin University, Changchun 130021, China; Jilin Provincial Key Laboratory of Radiation Oncology & Therapy, The First Hospital of Jilin University, Changchun 130021, China; NHC Key Laboratory of Radiobiology, School of Public Health, Jilin University, Changchun 130021, China.
| |
Collapse
|
|
12
|
Marcone S, Buckley A, Ryan CJ, McCabe M, Lynam-Lennon N, Matallanas D, O Sullivan J, Kennedy S. Proteomic signatures of radioresistance: Alteration of inflammation, angiogenesis and metabolism-related factors in radioresistant oesophageal adenocarcinoma. Cancer Treat Res Commun 2021; 27:100376. [PMID: 33882379 DOI: 10.1016/j.ctarc.2021.100376] [Citation(s) in RCA: 2] [Impact Index Per Article: 0.7] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/10/2021] [Revised: 04/09/2021] [Accepted: 04/11/2021] [Indexed: 01/06/2023]
Abstract
The clinical management of locally advanced oesophageal adenocarcinoma (OAC) involves neoadjuvant chemoradiotherapy (CRT), but as radioresistance remains a major clinical challenge, complete pathological response to CRT only occurs in 20-30% of patients. In this study we used an established isogenic cell line model of radioresistant OAC to detect proteomic signatures of radioresistance to identify novel molecular and cellular targets of radioresistance in OAC. A total of 5785 proteins were identified of which 251 were significantly modulated in OE33R cells, when compared to OE33P. Gene ontology and pathway analysis of these significantly modulated proteins demonstrated altered metabolism in radioresistant cells accompanied by an inhibition of apoptosis. In addition, inflammatory and angiogenic pathways were positively regulated in radioresistant cells compared to the radiosensitive cells. In this study, we demonstrate, for the first time, a comprehensive proteomic profile of the established isogenic cell line model of radioresistant OAC. This analysis provides insights into the molecular and cellular pathways which regulate radioresistance in OAC. Furthermore, it identifies pathway specific signatures of radioresistance that will direct studies on the development of targeted therapies and personalised approaches to radiotherapy.
Collapse
Affiliation(s)
- Simone Marcone
- Department of Surgery, Trinity Translational Medicine Institute, Trinity St. James's Cancer Institute, St. James's Hospital, Trinity College Dublin, Dublin, Ireland.
| | - Amy Buckley
- Department of Surgery, Trinity Translational Medicine Institute, Trinity St. James's Cancer Institute, St. James's Hospital, Trinity College Dublin, Dublin, Ireland
| | - Colm J Ryan
- School of Computer Science, University College Dublin, Dublin 4, Ireland; Systems Biology Ireland, School of Medicine, University College Dublin, Dublin 4, Ireland
| | - Mark McCabe
- Department of Surgery, Trinity Translational Medicine Institute, Trinity St. James's Cancer Institute, St. James's Hospital, Trinity College Dublin, Dublin, Ireland
| | - Niamh Lynam-Lennon
- Department of Surgery, Trinity Translational Medicine Institute, Trinity St. James's Cancer Institute, St. James's Hospital, Trinity College Dublin, Dublin, Ireland
| | - David Matallanas
- Systems Biology Ireland, School of Medicine, University College Dublin, Dublin 4, Ireland
| | - Jacintha O Sullivan
- Department of Surgery, Trinity Translational Medicine Institute, Trinity St. James's Cancer Institute, St. James's Hospital, Trinity College Dublin, Dublin, Ireland
| | - Susan Kennedy
- Department of Surgery, Trinity Translational Medicine Institute, Trinity St. James's Cancer Institute, St. James's Hospital, Trinity College Dublin, Dublin, Ireland
| |
Collapse
|
|
13
|
Sun X, Ni N, Ma Y, Wang Y, Leong DT. Retooling Cancer Nanotherapeutics' Entry into Tumors to Alleviate Tumoral Hypoxia. SMALL (WEINHEIM AN DER BERGSTRASSE, GERMANY) 2020; 16:e2003000. [PMID: 32803846 DOI: 10.1002/smll.202003000] [Citation(s) in RCA: 28] [Impact Index Per Article: 7.0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Subscribe] [Scholar Register] [Received: 05/13/2020] [Revised: 06/20/2020] [Indexed: 06/11/2023]
Abstract
Anti-hypoxia cancer nanomedicine (AHCN) holds exciting potential in improving oxygen-dependent therapeutic efficiencies of malignant tumors. However, most studies regarding AHCN focus on optimizing structure and function of nanomaterials with presupposed successful entry into tumor cells. From such a traditional perspective, the main barrier that AHCN needs to overcome is mainly the tumor cell membrane. However, such an oversimplified perspective would neglect that real tumors have many biological, physiological, physical, and chemical defenses preventing the current state-of-the-art AHCNs from even reaching the targeted tumor cells. Fortunately, in recent years, some studies are beginning to intentionally focus on overcoming physiological barriers to alleviate hypoxia. In this Review, the limitations behind the traditional AHCN delivery mindset are addressed and the key barriers that need to be surmounted before delivery to cancer cells and some good ways to improve cell membrane attachment, internalization, and intracellular retention are summarized. It is aimed to contribute to Review literature on this emerging topic through refreshing perspectives based on this work and what is also learnt from others. This Review would therefore assist AHCNs researchers to have a quick overview of the essential information and glean thought-provoking ideas to advance this sub-field in cancer nanomedicine.
Collapse
Affiliation(s)
- Xiao Sun
- Department of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, Singapore, 117585, Singapore
| | - Nengyi Ni
- Department of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, Singapore, 117585, Singapore
| | - Yanling Ma
- Department of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, Singapore, 117585, Singapore
| | - Yan Wang
- Department of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, Singapore, 117585, Singapore
| | - David Tai Leong
- Department of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, Singapore, 117585, Singapore
| |
Collapse
|
|
14
|
Buckley AM, Lynam-Lennon N, O'Neill H, O'Sullivan J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol Hepatol 2020; 17:298-313. [PMID: 32005946 DOI: 10.1038/s41575-019-0247-2] [Citation(s) in RCA: 156] [Impact Index Per Article: 39.0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Accepted: 11/26/2019] [Indexed: 12/19/2022]
Abstract
Radiotherapy is used in the treatment of approximately 50% of all malignancies including gastrointestinal cancers. Radiation can be given prior to surgery (neoadjuvant radiotherapy) to shrink the tumour or after surgery to kill any remaining cancer cells. Radiotherapy aims to maximize damage to cancer cells, while minimizing damage to healthy cells. However, only 10-30% of patients with rectal cancer or oesophageal cancer have a pathological complete response to neoadjuvant chemoradiation therapy, with the rest suffering the negative consequences of toxicities and delays to surgery with no clinical benefit. Furthermore, in pancreatic cancer, neoadjuvant chemoradiation therapy results in a pathological complete response in only 4% of patients and a partial pathological response in only 31%. Resistance to radiation therapy is polymodal and associated with a number of biological alterations both within the tumour itself and in the surrounding microenvironment including the following: altered cell cycle; repopulation by cancer stem cells; hypoxia; altered management of oxidative stress; evasion of apoptosis; altered DNA damage response and enhanced DNA repair; inflammation; and altered mitochondrial function and cellular energetics. Radiosensitizers are needed to improve treatment response to radiation, which will directly influence patient outcomes in gastrointestinal cancers. This article reviews the literature to identify strategies - including DNA-targeting agents, antimetabolic agents, antiangiogenics and novel immunotherapies - being used to enhance radiosensitivity in gastrointestinal cancers according to the hallmarks of cancer. Evidence from radiosensitizers from in vitro and in vivo models is documented and the action of radiosensitizers through clinical trial data is assessed.
Collapse
Affiliation(s)
- Amy M Buckley
- Department of Surgery, Trinity Translational Medicine Institute, St. James's Hospital, Trinity College Dublin, Dublin, Ireland
| | - Niamh Lynam-Lennon
- Department of Surgery, Trinity Translational Medicine Institute, St. James's Hospital, Trinity College Dublin, Dublin, Ireland
| | - Hazel O'Neill
- Department of Surgery, Trinity Translational Medicine Institute, St. James's Hospital, Trinity College Dublin, Dublin, Ireland
| | - Jacintha O'Sullivan
- Department of Surgery, Trinity Translational Medicine Institute, St. James's Hospital, Trinity College Dublin, Dublin, Ireland.
| |
Collapse
|
|
15
|
Ma C, Zhou J, Xu X, Wang L, Qin S, Hu C, Nie L, Tu Y. The Construction of a Radiation-Induced Brain Injury Model and Preliminary Study on the Effect of Human Recombinant Endostatin in Treating Radiation-Induced Brain Injury. Med Sci Monit 2019; 25:9392-9401. [PMID: 31816619 PMCID: PMC6921694 DOI: 10.12659/msm.917537] [Citation(s) in RCA: 5] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 12/13/2022] Open
Abstract
Background The aim of this study was to construct a radiation-induced brain injury (RBI) model and assess the effects of human recombinant endostatin in the treatment of RBI. Material/Methods In this study, the RBI model was used. Real-time quantitative polymerase chain reaction, immunohistochemistry, hematoxylin and eosin staining were conducted to detect the mRNA and protein expression of vascular endothelial growth factor (VEGF) and assess the effects of human recombinant endostatin in the treatment of RBI. Results In this study, we successfully constructed a RBI model. VEGF mRNA expression was decreased after human recombinant endostatin treatment; however, VEGF protein secretion was increased in brain endothelial cells, and the secretion of VEGF protein was decreased in glial cells and nerve cells. Body weight changes indicated that human recombinant endostatin can increase the risk of weight loss. Brain water content results showed that human recombinant endostatin might aggravate cerebral edema in the acute stage of RBI, but it can reduce the progression of cerebral edema in the early delayed stage. Survival analysis showed that human recombinant endostatin improved the survival rate only in the early stage of RBI. Conclusions Radiation can induce vasogenic edema and is associated with the RBI occurrence and development. VEGF protein is highly relevant to the induction of edema and thrombosis in the acute phase of RBI and in the early delayed phase of RBI, including vascular repair and regeneration, thrombus ablation and other events. Human recombinant endostatin can reduce the progression of cerebral edema during the early onset of RBI.
Collapse
Affiliation(s)
- Chenying Ma
- Department of Radiotherapy, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China (mainland)
| | - Juying Zhou
- Department of Radiotherapy, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China (mainland)
| | - Xiaoting Xu
- Department of Radiotherapy, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China (mainland)
| | - Lili Wang
- Department of Radiotherapy, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China (mainland)
| | - Songbin Qin
- Department of Radiotherapy, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China (mainland)
| | - Chao Hu
- Department of Radiotherapy, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China (mainland)
| | - Liangqin Nie
- Department of Radiotherapy, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China (mainland)
| | - Yu Tu
- School of Radiation Medicine and Protection, Medical College of Soochow University, Suzhou, Jiangsu, China (mainland)
| |
Collapse
|
|
16
|
Effects of transplantation of hypoxia-inducible factor-1α genemodified cardiac stem cells on cardiac function of heart failure rats after myocardial infarction. Anatol J Cardiol 2019; 20:318-329. [PMID: 30504732 PMCID: PMC6287433 DOI: 10.14744/anatoljcardiol.2018.91979] [Citation(s) in RCA: 4] [Impact Index Per Article: 0.8] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 01/25/2023] Open
Abstract
OBJECTIVE To evaluate the effects of transplantation of hypoxia-inducible factor-1α (HIF-1α) gene-modified cardiac stem cells (CSCs) on the cardiac function of heart failure rats after myocardial infarction (MI). METHODS Twenty-four Sprague-Dawley rats were randomly divided into three groups: HIF-1α-modified CSCs group, single CSCs group, and model group. The model of heart failure after MI was established by thoracotomy-left anterior descending coronary artery ligation, followed by injection of modified CSCs, single CSCs, and PBS, respectively, 2 weeks later. The results were observed 4 weeks later. RESULTS CSCs were infected with recombinant adenovirus. HIF-1α mRNA level of HIF-1α-enhanced green fluorescent protein (EGFP)+CSCs group significantly surpassed those of EGFP+CSCs and CSCs groups (p<0.001). Left ventricular ejection fractions (LVEFs) of HIF-1α+CSCs+MI and CSCs+MI groups significantly increased compared with the model group (p<0.001). The difference between LVEFs before and after transplantation was positively correlated with the survival rate of CSCs in infarction border zone (r=0.867, p<0.001). The apoptosis rate of HIF1α+CSCs+MI group was significantly lower than that of CSCs+MI group (p=0.008). The expression of vascular endothelial growth factor protein in HIF-1α+CSCs+MI group significantly increased, compared with that of MI group (p<0.001). The capillary density of HIF-1α+CSCs+MI group significantly exceeded that of CSCs+MI group (p<0.001). CONCLUSION Transplantation of either HIF-1α-modified CSCs or single CSCs reduced cardiomyocyte apoptosis in rats with heart failure after MI, promoted vascular regeneration in infarct area, and improved cardiac function. Particularly, HIF-1α-modified CSCs had more significant effects.
Collapse
|
|
17
|
Yang L, Xu Y, Luo P, Chen S, Zhu H, Wang C. Baseline platelet counts and derived inflammatory biomarkers: prognostic relevance in metastatic melanoma patients receiving Endostar plus dacarbazine and cisplatin. Cancer Manag Res 2019; 11:3681-3690. [PMID: 31118790 PMCID: PMC6500443 DOI: 10.2147/cmar.s194176] [Citation(s) in RCA: 6] [Impact Index Per Article: 1.2] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/11/2018] [Accepted: 04/01/2019] [Indexed: 01/19/2023] Open
Abstract
Background: The clinical efficacy and safety of Endostar combined with chemotherapy in the treatment of metastatic malignant melanoma (MM) were analyzed and the indicators capable of predicting the efficacy of the regimen were identified to guide clinical practice. Patients and methods: The clinical data of 55 patients with metastatic MM without gene mutations who were treated with Endostar combined with dacarbazine and cisplatin were retrospectively analyzed. Efficacy was assessed using RECIST 1.1, and adverse events (AEs) were graded according to NCI-CTCAE 4.0. The log-rank test was used to compare the survival curves of patients in different subgroups, and stepwise multivariate Cox regression analysis was used to determine significant prognostic factors. Differences were considered statistically significant at P<0.05. Results: Of the 55 patients, seven showed a partial response, 20 showed stable disease, and 28 showed progressive disease. The median progression-free survival was 17.9 months. AEs were controllable. Univariate analysis identified biotherapy, clinical stage, clinical classification, low baseline platelet count, platelet to albumin ratio (PAR), and platelet to globulin ratio (PGR) as factors affecting drug efficacy. Multivariate Cox regression analysis identified clinical stage and PAR as independent factors predicting the efficacy of the regimen. Conclusions: Endostar combined with chemotherapy showed a curative effect on metastatic MM without gene mutations, and AEs were controllable. The baseline platelet count and derived PAR and PGR values were associated with the efficacy of the regimen. The potential value of efficacy prediction remains to be further verified by prospective random experiments.
Collapse
Affiliation(s)
- Lingge Yang
- Department of Musculoskeletal Oncology, Fudan University Shanghai Cancer Center, Shanghai, People's Republic of China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, People's Republic of China
| | - Yu Xu
- Department of Musculoskeletal Oncology, Fudan University Shanghai Cancer Center, Shanghai, People's Republic of China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, People's Republic of China
| | - Peng Luo
- Department of Musculoskeletal Oncology, Fudan University Shanghai Cancer Center, Shanghai, People's Republic of China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, People's Republic of China
| | - Shiqi Chen
- Department of Musculoskeletal Oncology, Fudan University Shanghai Cancer Center, Shanghai, People's Republic of China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, People's Republic of China
| | - Huiyan Zhu
- Department of Musculoskeletal Oncology, Fudan University Shanghai Cancer Center, Shanghai, People's Republic of China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, People's Republic of China
| | - Chunmeng Wang
- Department of Musculoskeletal Oncology, Fudan University Shanghai Cancer Center, Shanghai, People's Republic of China.,Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, People's Republic of China
| |
Collapse
|
|
18
|
Li Y, Huang P, Peng H, Yue H, Wu M, Liu S, Qin R, Fan J, Han Y. Antitumor effects of Endostar(rh-endostatin) combined with gemcitabine in different administration sequences to treat Lewis lung carcinoma. Cancer Manag Res 2019; 11:3469-3479. [PMID: 31114380 PMCID: PMC6497885 DOI: 10.2147/cmar.s192868] [Citation(s) in RCA: 8] [Impact Index Per Article: 1.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/31/2018] [Accepted: 03/14/2019] [Indexed: 01/12/2023] Open
Abstract
Background: Endostatin therapy is known to efficiently inhibit angiogenesis and growth of endothelial cells. Nonetheless, the antitumor mechanisms of endostatin combined with chemotherapy remain to be elucidated. Methods: In our study, a Lewis lung carcinoma transplant mouse model was established and treated with the recombinant human [rh]-endostatin, Endostar, combined with gemcitabine at different sequences. 18F-FDG PET/CT imaging was performed to monitor tumor growth, and hypoxia was examined using an oxygen microelectrode. Vascular endothelial growth factor (VEGF) and alpha smooth muscle actin (α-SMA) levels were detected via immunohistochemistry analysis and cell cycle distributions were analyzed by flow cytometry. Results: Endostar decreased VEGF expression, improved hypoxia, and influenced cell cycle distributions. Simultaneous treatment of Endostar and gemcitabine displayed significantly tumor inhibition, possessed the lowest uptake of FDG, improved oxygen partial pressure, decreased expression of VEGF, and increased pericyte coverage. Cell cycle analysis demonstrated that cells accumulated in the S phase following gemcitabine treatment and G0/G1 arrest occurred following Endostar treatment. An increase of cells in G0/G1 phase was observed following treatment with Endostar and gemcitabine. Conclusions: Our study suggests that the combination therapy of Endostar with gemcitabine simutaneously may optimally enhance their individual antitumor effects.
Collapse
Affiliation(s)
- Yuan Li
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Pan Huang
- Neurology Department, Deyang People's Hospital, Deyang, Sichuan 618000, People's Republic of China
| | - Hongju Peng
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Hongcheng Yue
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Min Wu
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Shanshan Liu
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Rongsheng Qin
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Juan Fan
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Yunwei Han
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| |
Collapse
|
|
19
|
Pyrazinib (P3), [(E)-2-(2-Pyrazin-2-yl-vinyl)-phenol], a small molecule pyrazine compound enhances radiosensitivity in oesophageal adenocarcinoma. Cancer Lett 2019; 447:115-129. [DOI: 10.1016/j.canlet.2019.01.009] [Citation(s) in RCA: 12] [Impact Index Per Article: 2.4] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/01/2018] [Revised: 11/13/2018] [Accepted: 01/07/2019] [Indexed: 02/06/2023]
|
|
20
|
Pan JH, Zhu S, Huang J, Liang J, Zhang D, Zhao X, Ding H, Qin L, Shi C, Luo L, Pan Y. Monitoring the Process of Endostar-Induced Tumor Vascular Normalization by Non-contrast Intravoxel Incoherent Motion Diffusion-Weighted MRI. Front Oncol 2018; 8:524. [PMID: 30483478 PMCID: PMC6243029 DOI: 10.3389/fonc.2018.00524] [Citation(s) in RCA: 20] [Impact Index Per Article: 3.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/17/2018] [Accepted: 10/25/2018] [Indexed: 01/26/2023] Open
Abstract
Tumor vascular normalization has been proposed as a new concept in anti-tumor angiogenesis, and the normalization window is considered as an opportunity to increase the effect of chemoradiotherapy. However, there is still a lack of a non-invasive method for monitoring the process of tumor vascular normalization. Intravoxel incoherent motion diffusion-weighted magnetic resonance imaging (IVIM DW-MRI) is an emerging approach which can effectively assess microperfusion in tumors, without the need for exogenous contrast agents. However, its role in monitoring tumor vascular normalization still needs further study. In this study, we established a tumor vascular normalization model of CT26 colon-carcinoma-bearing mice by means of Endostar treatment. We then employed IVIM DW-MRI and immunofluorescence to detect the process of tumor vascular normalization at different times after treatment. We found that the D* values of the Endostar group were significantly higher than those of the control group on days 4, 6, 8, and 10 after treatment, and the f values of the Endostar group were significantly higher than those of the control group on days 6 and 8. Furthermore, we confirmed through analysis of histologic parameters that Endostar treatment induced the CT26 tumor vascular normalization window starting from day 4 after treatment, and this window lasted for 6 days. Moreover, we found that D* and f values were well correlated with pericyte coverage (r = 0.469 and 0.504, respectively; P < 0.001, both) and relative perfusion (r = 0.424 and 0.457, respectively; P < 0.001, both). Taken together, our findings suggest that IVIM DW-MRI has the potential to serve as a non-invasive approach for monitoring Endostar-induced tumor vascular normalization.
Collapse
Affiliation(s)
- Jing-Hua Pan
- Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Shengbin Zhu
- Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Jinlian Huang
- Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Jianye Liang
- Medical Imaging Center, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Dong Zhang
- Medical Imaging Center, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Xiaoxu Zhao
- Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Hui Ding
- Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Li Qin
- Department of Histology and Embryology, Medical School of Jinan University, Guangzhou, China
| | - Changzheng Shi
- Medical Imaging Center, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Liangping Luo
- Medical Imaging Center, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Yunlong Pan
- Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| |
Collapse
|
|
21
|
Anti-tumor effect of endostatin in a sleep-apnea mouse model with tumor. Clin Transl Oncol 2018; 21:572-581. [PMID: 30293229 DOI: 10.1007/s12094-018-1955-8] [Citation(s) in RCA: 15] [Impact Index Per Article: 2.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/06/2018] [Accepted: 09/11/2018] [Indexed: 12/14/2022]
Abstract
BACKGROUND Obstructive sleep apnea (OSA) is associated with cancer incidence and mortality. The underlying mechanism is unclear. This study aims to evaluate the influence of intermittent hypoxia (IH), a novel hallmark of OSA, on tumor and to access the anti-tumor effect of endostatin on a mouse model with OSA. METHODS The C57BL/6 J mice were randomly classified into four groups: control (normoxia) (CTL), control plus endostatin (CTL + ED), IH, and IH plus endostatin (IH + ED). Mice in IH and IH + ED groups were subjected to IH 8 h per day in 5 weeks. Lewis lung cancer cells were injected into the flank of each mouse after 1 week of IH exposure. Endostatin was also intraperitoneally injected after tumor volume reached about 200 mm3. The maximum standard uptake values (SUVmax) were detected by micro-positron emission tomography-computed tomography (micro-PET-CT) imaging prior and post-endostatin administration. Microvessel density (MVD) and vascular endothelial growth factor (VEGF) were determined for evaluating the anti-tumor effect of endostatin among the normoxia and IH conditions. RESULTS Mice had higher SUVmax in the IH group than the CTL group (p < 0.01). When compared with mice in the CTL group, those in the IH group had significantly greater MVD values (p < 0.001). The SUVmax can be attenuated by endostatin both in the CTL (p < 0.01) and IH conditions (p < 0.001). When compared with CTL group, mice in the IH group had increased MVD values (p < 0.001) and VEGF expression both at mRNA (p < 0.05) and protein levels (p < 0.001 in western blotting results). Treatment with endostatin attenuated serum and tissue VEGF levels, lowering the MVD values. As compared to normoxia condition, the endostatin-therapeutic effects were more significant under the IH condition (p < 0.05 in western blotting results). CONCLUSIONS Micro-PET-CT imaging is a promising non-invasive technique to evaluate the tumor metabolic characteristics under IH condition in vivo. The anti-tumor effect of endostatin under IH condition is superior to that of the normoxia condition.
Collapse
|
|
22
|
Zhu S, Gu Z, Zhao Y. Harnessing Tumor Microenvironment for Nanoparticle-Mediated Radiotherapy. ADVANCED THERAPEUTICS 2018. [DOI: 10.1002/adtp.201800050] [Citation(s) in RCA: 27] [Impact Index Per Article: 4.5] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 12/12/2022]
Affiliation(s)
- Shuang Zhu
- CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety; Institute of High Energy Physics; Chinese Academy of Sciences; Beijing 100049 China
| | - Zhanjun Gu
- CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety; Institute of High Energy Physics; Chinese Academy of Sciences; Beijing 100049 China
- College of Materials Science and Optoelectronic Technology; University of Chinese Academy of Sciences; Beijing 100049 China
| | - Yuliang Zhao
- CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety; Institute of High Energy Physics; Chinese Academy of Sciences; Beijing 100049 China
- CAS Center for Excellence in Nanoscience; National Center for Nanoscience and Technology of China; Chinese Academy of Sciences; Beijing 100190 China
- College of Materials Science and Optoelectronic Technology; University of Chinese Academy of Sciences; Beijing 100049 China
| |
Collapse
|
|
23
|
He L, Zhao C, Li Y, Du G, Liu K, Cui D, Tang L, Wu X, Wen S, Chen H. Antiangiogenic effects of recombinant human endostatin in lung cancers. Mol Med Rep 2017; 17:79-86. [PMID: 29115591 PMCID: PMC5780156 DOI: 10.3892/mmr.2017.7859] [Citation(s) in RCA: 2] [Impact Index Per Article: 0.3] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/15/2016] [Accepted: 08/22/2017] [Indexed: 12/31/2022] Open
Abstract
Antiangiogenic therapy, as a new anticancer method, can improve the anticancer effect of traditional therapies. Different antiangiogenic drugs may have different vascular normalization time windows. Whether the antiangiogenic treatment is within the vascular normalization time window is very important in the treatment of cancers. Previous studies have indicated that recombinant human endostatin (rh-ES) can transiently normalize tumor microvessels. Yet the molecular mechanism and the time window of rh-ES remains unclear. The aim of the present study was to explore the optimal time window and molecular mechanism of rh-ES in inhibiting Lewis lung cancer (LLC). By comparatively accessing the changes in microvascular and hypoxic conditions of tumors in host mice treated with rh-ES or saline for different days, the authors aimed to investigate the best administration time of rh-ES treatment on human lung cancers and obtain a better understanding concerning the involved molecular mechanism. A total of 40 C57/BL6 mice with LLC xenografts were randomly divided into normal saline (NS) and rh-ES groups (20 mice/group). 0.2 ml NS or 5 mg/kg rh-ES were administrated via intraperitoneal injection (i.p.) into each mouse each day during the 9-day experiment. A total of 5 mice from each group were sacrificed at day 2, 4, 6 or 9. CA9 and RGS5 expression levels of both groups were compared using immunohistochemistry, reverse transcription-quantitative polymerase chain reaction and ELISA. Rh-ES caused vascular normalization and improved hypoxia at days 4 and 6. Compared with the control (NS) group, both CA9 and RGS5 expression in rh-ES group were significantly decreased at days 4 and 6 (P<0.05), while no significant change between two groups was observed at days 2 and 9. Rh-ES can induce transient tumor vascular normalization and improves tissue hypoxia in LLC tumors. The vascular normalization window is accompanied by the reduction in RGS5 and CA expression.
Collapse
Affiliation(s)
- Lang He
- Department of Oncology, The Fifth People's Hospital of Chengdu, North Sichuan Medical College, Chengdu, Sichuan 611130, P.R. China
| | - Chaofen Zhao
- Department of Medical Oncology, Affiliated Hospital of Guizhou Medical University, Guizhou Cancer Hospital, Guiyang, Guizhou 550004, P.R. China
| | - Yunxiang Li
- Department of Urology, The Second Clinical Medical College of North Sichuan Medical College, Nanchong, Sichuan 637000, P.R. China
| | - Guocheng Du
- Department of Galactophore, The Second Clinical Medical College of North Sichuan Medical College, Nanchong, Sichuan 637000, P.R. China
| | - Kang Liu
- Institute of Tissue Engineering and Stem Cells, North Sichuan Medical College, Nanchong, Sichuan 637000, P.R. China
| | - Dandan Cui
- Department of Oncology, The Fifth People's Hospital of Chengdu, North Sichuan Medical College, Chengdu, Sichuan 611130, P.R. China
| | - Lina Tang
- Department of Clinical Medicine, North Sichuan Medical College, Nanchong, Sichuan 637000, P.R. China
| | - Xun Wu
- Department of Clinical Medicine, North Sichuan Medical College, Nanchong, Sichuan 637000, P.R. China
| | - Shimin Wen
- Cancer Center, The Second Clinical Medical College of North Sichuan Medical College, Nanchong, Sichuan 637000, P.R. China
| | - Hong Chen
- Tumor Department of TCM, Sichuan Cancer Hospital and Institute, Chengdu, Sichuan 610000, P.R. China
| |
Collapse
|
|
24
|
Pan F, Yang W, Li W, Yang XY, Liu S, Li X, Zhao X, Ding H, Qin L, Pan Y. Conjugation of gold nanoparticles and recombinant human endostatin modulates vascular normalization via interruption of anterior gradient 2-mediated angiogenesis. Tumour Biol 2017; 39:1010428317708547. [PMID: 28714365 DOI: 10.1177/1010428317708547] [Citation(s) in RCA: 23] [Impact Index Per Article: 3.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 01/21/2023] Open
Abstract
Several studies have revealed the potential of normalizing tumor vessels in anti-angiogenic treatment. Recombinant human endostatin is an anti-angiogenic agent which has been applied in clinical tumor treatment. Our previous research indicated that gold nanoparticles could be a nanoparticle carrier for recombinant human endostatin delivery. The recombinant human endostatin-gold nanoparticle conjugates normalized vessels, which improved chemotherapy. However, the mechanism of recombinant human endostatin-gold nanoparticle-induced vascular normalization has not been explored. Anterior gradient 2 has been reported to be over-expressed in many malignant tumors and involved in tumor angiogenesis. To date, the precise efficacy of recombinant human endostatin-gold nanoparticles on anterior gradient 2-mediated angiogenesis or anterior gradient 2-related signaling cohort remained unknown. In this study, we aimed to explore whether recombinant human endostatin-gold nanoparticles could normalize vessels in metastatic colorectal cancer xenografts, and we further elucidated whether recombinant human endostatin-gold nanoparticles could interrupt anterior gradient 2-induced angiogenesis. In vivo, it was indicated that recombinant human endostatin-gold nanoparticles increased pericyte expression while inhibit vascular endothelial growth factor receptor 2 and anterior gradient 2 expression in metastatic colorectal cancer xenografts. In vitro, we uncovered that recombinant human endostatin-gold nanoparticles reduced cell migration and tube formation induced by anterior gradient 2 in human umbilical vein endothelial cells. Treatment with recombinant human endostatin-gold nanoparticles attenuated anterior gradient 2-mediated activation of MMP2, cMyc, VE-cadherin, phosphorylation of p38, and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) in human umbilical vein endothelial cells. Our findings demonstrated recombinant human endostatin-gold nanoparticles might normalize vessels by interfering anterior gradient 2-mediated angiogenesis in metastatic colorectal cancer.
Collapse
Affiliation(s)
- Fan Pan
- 1 Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Wende Yang
- 1 Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Wei Li
- 1 Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Xiao-Yan Yang
- 1 Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Shuhao Liu
- 1 Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Xin Li
- 1 Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Xiaoxu Zhao
- 1 Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Hui Ding
- 1 Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| | - Li Qin
- 2 Department of Histology and Embryology, Medical School of Jinan University, Guangzhou, China
| | - Yunlong Pan
- 1 Department of General Surgery, The First Affiliated Hospital of Jinan University, Guangzhou, China
| |
Collapse
|
|
25
|
Chen GZ, Zhu HC, Dai WS, Zeng XN, Luo JH, Sun XC. The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity. J Thorac Dis 2017; 9:849-859. [PMID: 28449496 PMCID: PMC5394057 DOI: 10.21037/jtd.2017.03.23] [Citation(s) in RCA: 56] [Impact Index Per Article: 8.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/07/2016] [Accepted: 01/19/2017] [Indexed: 12/21/2022]
Abstract
Esophageal cancer is the eighth most common cancer and the sixth leading cause of cancer-related death worldwide. Surgery is the primary form of treatment, but the survival is poor, especially for patients with locally advanced esophageal cancer. Radiotherapy has been a critical treatment option that may be combined with chemotherapy in patients with unresectable esophageal cancer. However, resistance to chemoradiotherapy might result in treatment failures and cancer relapse. This review will mainly focus on the possible cellular mechanisms and tumor-associated microenvironmental (TAM) factors that result in radioresistance in patients with esophageal cancer. In addition, current strategies to increase radiosensitivity, including targeted therapy and the use of radiosensitive biomarkers in clinical treatment, are discussed in this review.
Collapse
Affiliation(s)
- Guang-Zong Chen
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Hong-Cheng Zhu
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Wang-Shu Dai
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Xiao-Ning Zeng
- Department of Respiratory Medicine, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Jin-Hua Luo
- Department of Thoracic Surgery, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Xin-Chen Sun
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| |
Collapse
|
|
26
|
Lin CW, Wang LK, Wang SP, Chang YL, Wu YY, Chen HY, Hsiao TH, Lai WY, Lu HH, Chang YH, Yang SC, Lin MW, Chen CY, Hong TM, Yang PC. Daxx inhibits hypoxia-induced lung cancer cell metastasis by suppressing the HIF-1α/HDAC1/Slug axis. Nat Commun 2016; 7:13867. [PMID: 28004751 PMCID: PMC5192219 DOI: 10.1038/ncomms13867] [Citation(s) in RCA: 63] [Impact Index Per Article: 7.9] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 02/22/2016] [Accepted: 11/07/2016] [Indexed: 12/20/2022] Open
Abstract
Hypoxia is a major driving force of cancer invasion and metastasis. Here we show that death domain-associated protein (Daxx) acts to negatively regulate hypoxia-induced cell dissemination and invasion by inhibiting the HIF-1α/HDAC1/Slug pathway. Daxx directly binds to the DNA-binding domain of Slug, impeding histone deacetylase 1 (HDAC1) recruitment and antagonizing Slug E-box binding. This, in turn, stimulates E-cadherin and occludin expression and suppresses Slug-mediated epithelial–mesenchymal transition (EMT) and cell invasiveness. Under hypoxic conditions, stabilized hypoxia-inducible factor (HIF)-1α downregulates Daxx expression and promotes cancer invasion, whereas re-expression of Daxx represses hypoxia-induced cancer invasion. Daxx also suppresses Slug-mediated lung cancer metastasis in an orthotopic lung metastasis mouse model. Using clinical tumour samples, we confirmed that the HIF-1α/Daxx/Slug pathway is an outcome predictor. Our results support that Daxx can act as a repressor in controlling HIF-1α/HDAC1/Slug-mediated cancer cell invasion and is a potential therapeutic target for inhibition of cancer metastasis. Hypoxia and epithelial-to-mesenchymal transition promotes cancer metastasis. Here the authors show that Daxx inhibits hypoxia-induced lung cancer metastasis by attenuating Slug-mediated transcriptional repression of epithelial-like markers that in turn cause cells to exhibit low invasiveness.
Collapse
Affiliation(s)
- Ching-Wen Lin
- Institute of Biomedical Sciences, Academia Sinica, Taipei 11529, Taiwan
| | - Lu-Kai Wang
- Radiation Biology Core Laboratory of Institute for Radiological Research, Chang Gung University/Chang Gung Memorial Hospital, Taoyuan 33305, Taiwan
| | - Shu-Ping Wang
- Laboratory of Biochemistry and Molecular Biology, The Rockefeller University, New York, New York 10065, USA
| | - Yi-Liang Chang
- Department of Pathology and Graduate Institute of Pathology, National Taiwan University College of Medicine, Taipei 10051, Taiwan
| | - Yi-Ying Wu
- Graduate Institute of Clinical Medicine, National Cheng Kung University, Tainan 70101, Taiwan
| | - Hsuan-Yu Chen
- Institute of Statistical Science, Academia Sinica, Taipei 11529, Taiwan
| | - Tzu-Hung Hsiao
- Department of Medical Research, Taichung Veterans General Hospital, Taichung 40705, Taiwan
| | - Wei-Yun Lai
- Aptamer Core, Institute of Biomedical Sciences, Academia Sinica, Taipei 11529, Taiwan
| | - Hsuan-Hsuan Lu
- Department of Internal Medicine, National Taiwan University College of Medicine, Taipei 10051, Taiwan
| | - Ya-Hsuan Chang
- Institute of Statistical Science, Academia Sinica, Taipei 11529, Taiwan
| | - Shuenn-Chen Yang
- Institute of Biomedical Sciences, Academia Sinica, Taipei 11529, Taiwan
| | - Ming-Wei Lin
- Program in Molecular Medicine, National Yang-Ming University and Academia Sinica, Taipei 11221, Taiwan
| | - Chi-Yuan Chen
- Graduate Institute of Health Industry Technology and Research Center for Industry of Human Ecology, College of Human Ecology, Chang Gung University of Science and Technology, Taoyuan 33303, Taiwan
| | - Tse-Ming Hong
- Graduate Institute of Clinical Medicine, National Cheng Kung University, Tainan 70101, Taiwan
| | - Pan-Chyr Yang
- Institute of Biomedical Sciences, Academia Sinica, Taipei 11529, Taiwan.,Department of Internal Medicine, National Taiwan University College of Medicine, Taipei 10051, Taiwan
| |
Collapse
|
|
27
|
Gold Nanoparticle-Mediated Targeted Delivery of Recombinant Human Endostatin Normalizes Tumour Vasculature and Improves Cancer Therapy. Sci Rep 2016; 6:30619. [PMID: 27470938 PMCID: PMC4965746 DOI: 10.1038/srep30619] [Citation(s) in RCA: 69] [Impact Index Per Article: 8.6] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 04/27/2016] [Accepted: 07/06/2016] [Indexed: 01/19/2023] Open
Abstract
Tumour vasculature is generally disordered because of the production of excessive angiogenic factors by tumour cells, which results in tumour progression and reduces the effectiveness of radiotherapy or chemotherapy. Transient anti-angiogenic therapies that regulate tumour vascular morphology and function and improve the efficiency of antitumour therapy are under investigation. Recombinant human endostatin (Endostar/rhES) is a vascular angiogenesis–disrupting agent that has been used to treat non-small cell lung cancer (NSCLC) in the clinical setting. In this study, we used gold nanoparticles (AuNPs) as a drug-delivery system (DDS) for targeted tumour delivery of rhES for short therapy, which resulted in transient tumour vascular normalization, reduced permeability and hypoxia, strengthened blood vessel integrity, and increased blood-flow perfusion. Moreover, combination therapy with 5-FU over this timeframe was substantially more effective than 5-FU monotherapy. In conclusion, our research demonstrates the potential use of AuNPs as a drug-delivery platform for transporting rhES into a tumour to induce transient tumour vascular normalization and enhance the antitumour efficacy of cytotoxic drugs.
Collapse
|
|
28
|
Endothelial Cell Response to Fusobacterium nucleatum. Infect Immun 2016; 84:2141-2148. [PMID: 27185790 DOI: 10.1128/iai.01305-15] [Citation(s) in RCA: 32] [Impact Index Per Article: 4.0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/19/2015] [Accepted: 05/06/2016] [Indexed: 12/19/2022] Open
Abstract
Vascular response is an essential aspect of an effective immune response to periodontal disease pathogens, as new blood vessel formation contributes to wound healing and inflammation. Gaining a greater understanding of the factors that affect vascular response may then contribute to future breakthroughs in dental medicine. In this study, we have characterized the endothelial cell response to the common bacterium Fusobacterium nucleatum, an important bridging species that facilitates the activity of late colonizers of the dental biofilm. Endothelial cells were infected with Fusobacterium nucleatum (strain 25586) for periods of 4, 12, 24, or 48 h. Cell proliferation and tube formation were analyzed, and expression of adhesion molecules (CD31 and CD34) and vascular endothelial growth factor (VEGF) receptors 1 and 2 was measured by fluorescence-activated cell sorter (FACS) analysis. Data indicate that F. nucleatum impaired endothelial cell proliferation and tube formation. The findings suggest that the modified endothelial cell response acts as a mechanism promoting the pathogenic progression of periodontal diseases and may potentially suggest the involvement of periodontopathogens in systemic diseases associated with periodontal inflammation.
Collapse
|
|
29
|
Ricard-Blum S, Vallet SD. Matricryptins Network with Matricellular Receptors at the Surface of Endothelial and Tumor Cells. Front Pharmacol 2016; 7:11. [PMID: 26869928 PMCID: PMC4740388 DOI: 10.3389/fphar.2016.00011] [Citation(s) in RCA: 43] [Impact Index Per Article: 5.4] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/20/2015] [Accepted: 01/12/2016] [Indexed: 12/11/2022] Open
Abstract
The extracellular matrix (ECM) is a source of bioactive fragments called matricryptins or matrikines resulting from the proteolytic cleavage of extracellular proteins (e.g., collagens, elastin, and laminins) and proteoglycans (e.g., perlecan). Matrix metalloproteinases (MMPs), cathepsins, and bone-morphogenetic protein-1 release fragments, which regulate physiopathological processes including tumor growth, metastasis, and angiogenesis, a pre-requisite for tumor growth. A number of matricryptins, and/or synthetic peptides derived from them, are currently investigated as potential anti-cancer drugs both in vitro and in animal models. Modifications aiming at improving their efficiency and their delivery to their target cells are studied. However, their use as drugs is not straightforward. The biological activities of these fragments are mediated by several receptor families. Several matricryptins may bind to the same matricellular receptor, and a single matricryptin may bind to two different receptors belonging or not to the same family such as integrins and growth factor receptors. Furthermore, some matricryptins interact with each other, integrins and growth factor receptors crosstalk and a signaling pathway may be regulated by several matricryptins. This forms an intricate 3D interaction network at the surface of tumor and endothelial cells, which is tightly associated with other cell-surface associated molecules such as heparan sulfate, caveolin, and nucleolin. Deciphering the molecular mechanisms underlying the behavior of this network is required in order to optimize the development of matricryptins as anti-cancer agents.
Collapse
Affiliation(s)
- Sylvie Ricard-Blum
- University Claude Bernard Lyon 1, UMR 5246 Centre National de la Recherche Scientifique - University Lyon 1 - Institut National des Sciences Appliquées de Lyon - École Supérieure de Chimie Physique Électronique de Lyon Villeurbanne, France
| | - Sylvain D Vallet
- University Claude Bernard Lyon 1, UMR 5246 Centre National de la Recherche Scientifique - University Lyon 1 - Institut National des Sciences Appliquées de Lyon - École Supérieure de Chimie Physique Électronique de Lyon Villeurbanne, France
| |
Collapse
|