|
1
|
Glowa C, Bendinger AL, Euler-Lange R, Peschke P, Brons S, Debus J, Karger CP. Irradiation with Carbon Ions Effectively Counteracts Hypoxia-related Radioresistance in a Rat Prostate Carcinoma. Int J Radiat Oncol Biol Phys 2024; 120:875-883. [PMID: 38750905 DOI: 10.1016/j.ijrobp.2024.05.004] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/08/2024] [Revised: 04/29/2024] [Accepted: 05/06/2024] [Indexed: 06/04/2024]
Abstract
PURPOSE Hypoxia in tumors is associated with increased malignancy and resistance to conventional photon radiation therapy. This study investigated the potential of particle therapy to counteract radioresistance in syngeneic rat prostate carcinoma. METHODS AND MATERIALS Subcutaneously transplanted R3327-HI tumors were irradiated with photons or carbon ions under acute hypoxic conditions, induced by clamping the tumor-supplying artery 10 min before and during irradiation. Dose-response curves were determined for the endpoint "local tumor control within 300 days" and compared with previously published data acquired under oxic conditions. Doses at 50% tumor control probability (TCD50) were used to quantify hypoxia-induced radioresistance relative to that under oxic conditions and also to quantify the increased effectiveness of carbon ions under oxic and hypoxic conditions relative to photons. RESULTS Compared with those under oxic conditions, TCD50 values under hypoxic conditions increased by a factor of 1.53 ± 0.08 for photons and by a factor of 1.28 ± 0.06 for carbon ions (oxygen enhancement ratio). Compared with those for photons, TCD50 values for carbon ions decreased by a factor of 2.08 ± 0.13 under oxic conditions and by a factor of 2.49 ± 0.08 under hypoxic conditions (relative biological effectiveness). While the slope of the photon dose-response curves increased when changing from oxic to hypoxic conditions, the slopes were steeper and remained unchanged for carbon ions. CONCLUSIONS The reduced oxygen enhancement ratio of carbon ions indicated that the required dose increase in hypoxic tumors was 17% lower for carbon ions than for photons. Additionally, carbon ions reduced the effect of intertumor heterogeneity on the radiation response. Therefore, carbon ions may confer a significant advantage for the treatment of hypoxic tumors that are highly resistant to conventional photon radiation therapy.
Collapse
Affiliation(s)
- Christin Glowa
- Department of Radiation Oncology and Radiotherapy, University Hospital Heidelberg, Heidelberg, Germany; Department of Medical Physics in Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany; Heidelberg Institute for Radiation Oncology (HIRO), National Center for Radiation Research in Oncology (NCRO), Heidelberg, Germany
| | - Alina L Bendinger
- Department of Medical Physics in Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany; Heidelberg Institute for Radiation Oncology (HIRO), National Center for Radiation Research in Oncology (NCRO), Heidelberg, Germany; University of Heidelberg, Faculty of Biosciences, Heidelberg, Germany
| | - Rosemarie Euler-Lange
- Department of Medical Physics in Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany; Heidelberg Institute for Radiation Oncology (HIRO), National Center for Radiation Research in Oncology (NCRO), Heidelberg, Germany; Department of Radiooncology/Radiobiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
| | - Peter Peschke
- Department of Medical Physics in Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany; Heidelberg Institute for Radiation Oncology (HIRO), National Center for Radiation Research in Oncology (NCRO), Heidelberg, Germany
| | - Stephan Brons
- Heidelberg Institute for Radiation Oncology (HIRO), National Center for Radiation Research in Oncology (NCRO), Heidelberg, Germany; Heidelberg Ion Beam Therapy Center (HIT), Heidelberg, Germany
| | - Jürgen Debus
- Department of Radiation Oncology and Radiotherapy, University Hospital Heidelberg, Heidelberg, Germany; Heidelberg Institute for Radiation Oncology (HIRO), National Center for Radiation Research in Oncology (NCRO), Heidelberg, Germany; Clinical Cooperation Unit Radiation Therapy, German Cancer Research Center (DKFZ), Heidelberg, Germany
| | - Christian P Karger
- Department of Medical Physics in Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany; Heidelberg Institute for Radiation Oncology (HIRO), National Center for Radiation Research in Oncology (NCRO), Heidelberg, Germany.
| |
Collapse
|
|
2
|
Zhang WX, Zhou ZL, Lv QY, Song X, Chen J, Niu CB, Cui HF. O 2-Generation-Enhanced Responsive Starvation/Photothermal Synergistic Tumor Therapy Based on the AuNRs@MnO 2@SiO 2 Nanocarrier and Thermosensitive Biomimetic Camouflaging. ACS APPLIED BIO MATERIALS 2023; 6:4775-4790. [PMID: 37830366 DOI: 10.1021/acsabm.3c00544] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 10/14/2023]
Abstract
Cancer starvation/photothermal combined tumor therapy (CST/PTT) has attracted great interest attributed to their mutual compensation and synergistically enhanced effect. However, the very low O2 supply in the tumor microenvironment (TME) greatly limits the CST efficiency of glucose oxidase (GOx). Additionally, the easy degradation in blood circulation and significant off-target effects are big challenges for clinical applications of the GOx-based CST. In this study, a drug delivery system (DDS) with specific tumor-targeted GOx delivery, near-infrared (NIR) light and TME responsive O2 generation, NIR-responsive glucose consumption, high GOx loading, and efficient NIR photothermia was developed. Positively charged AuNRs@MnO2@SiO2 nanoparticles (named AMS+ NPs) were synthesized. GOx was covalently loaded with a high loading ratio of 36.0%. Finally, a thermosensitive biomimetic hybrid membrane composed of a thermosensitive lipid (TSL) membrane, red blood cell membrane (RBCM), and 4T1 cancer cell membrane (CCM) was coated on the NPs through a double-layer strategy. The AMS+-G@TSL@[RBC-CC-TSL]M NPs consumed 32.7 times glucose at 50 °C as that at 37 °C and generated 4.9 times O2 upon NIR laser irradiation. The thermosensitive biomimetic NPs showed an efficient targeting capability to the homotypic 4T1 cancer cells/tumors accompanied by good biocompatibility, macrophage evading capability, high cancer cell cytotoxicity, and excellent antitumor efficacy. The tumor growth inhibition ratio with NIR laser irradiation reached 92.8%. The AMS+-GOx@TSL@[RBC-CC-TSL]M NPs provide a smart, efficient, safe, PTT/CST combined DDS for highly efficient tumor therapy.
Collapse
Affiliation(s)
- Wen-Xing Zhang
- School of Life Sciences, Zhengzhou University, Science Avenue 100#, Zhengzhou 450001, China
| | - Ze-Lei Zhou
- School of Life Sciences, Zhengzhou University, Science Avenue 100#, Zhengzhou 450001, China
| | - Qi-Yan Lv
- School of Life Sciences, Zhengzhou University, Science Avenue 100#, Zhengzhou 450001, China
| | - Xiejie Song
- School of Life Sciences, Zhengzhou University, Science Avenue 100#, Zhengzhou 450001, China
| | - Junyang Chen
- School of Life Sciences, Zhengzhou University, Science Avenue 100#, Zhengzhou 450001, China
| | - Chang-Bin Niu
- School of Life Sciences, Zhengzhou University, Science Avenue 100#, Zhengzhou 450001, China
| | - Hui-Fang Cui
- School of Life Sciences, Zhengzhou University, Science Avenue 100#, Zhengzhou 450001, China
| |
Collapse
|
|
3
|
Reyes-Aldasoro CC. Modelling the Tumour Microenvironment, but What Exactly Do We Mean by "Model"? Cancers (Basel) 2023; 15:3796. [PMID: 37568612 PMCID: PMC10416922 DOI: 10.3390/cancers15153796] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/28/2023] [Revised: 07/19/2023] [Accepted: 07/25/2023] [Indexed: 08/13/2023] Open
Abstract
The Oxford English Dictionary includes 17 definitions for the word "model" as a noun and another 11 as a verb. Therefore, context is necessary to understand the meaning of the word model. For instance, "model railways" refer to replicas of railways and trains at a smaller scale and a "model student" refers to an exemplary individual. In some cases, a specific context, like cancer research, may not be sufficient to provide one specific meaning for model. Even if the context is narrowed, specifically, to research related to the tumour microenvironment, "model" can be understood in a wide variety of ways, from an animal model to a mathematical expression. This paper presents a review of different "models" of the tumour microenvironment, as grouped by different definitions of the word into four categories: model organisms, in vitro models, mathematical models and computational models. Then, the frequencies of different meanings of the word "model" related to the tumour microenvironment are measured from numbers of entries in the MEDLINE database of the United States National Library of Medicine at the National Institutes of Health. The frequencies of the main components of the microenvironment and the organ-related cancers modelled are also assessed quantitatively with specific keywords. Whilst animal models, particularly xenografts and mouse models, are the most commonly used "models", the number of these entries has been slowly decreasing. Mathematical models, as well as prognostic and risk models, follow in frequency, and these have been growing in use.
Collapse
|
|
4
|
Ng J, Gregucci F, Pennell RT, Nagar H, Golden EB, Knisely JPS, Sanfilippo NJ, Formenti SC. MRI-LINAC: A transformative technology in radiation oncology. Front Oncol 2023; 13:1117874. [PMID: 36776309 PMCID: PMC9911688 DOI: 10.3389/fonc.2023.1117874] [Citation(s) in RCA: 5] [Impact Index Per Article: 5.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/07/2022] [Accepted: 01/16/2023] [Indexed: 01/28/2023] Open
Abstract
Advances in radiotherapy technologies have enabled more precise target guidance, improved treatment verification, and greater control and versatility in radiation delivery. Amongst the recent novel technologies, Magnetic Resonance Imaging (MRI) guided radiotherapy (MRgRT) may hold the greatest potential to improve the therapeutic gains of image-guided delivery of radiation dose. The ability of the MRI linear accelerator (LINAC) to image tumors and organs with on-table MRI, to manage organ motion and dose delivery in real-time, and to adapt the radiotherapy plan on the day of treatment while the patient is on the table are major advances relative to current conventional radiation treatments. These advanced techniques demand efficient coordination and communication between members of the treatment team. MRgRT could fundamentally transform the radiotherapy delivery process within radiation oncology centers through the reorganization of the patient and treatment team workflow process. However, the MRgRT technology currently is limited by accessibility due to the cost of capital investment and the time and personnel allocation needed for each fractional treatment and the unclear clinical benefit compared to conventional radiotherapy platforms. As the technology evolves and becomes more widely available, we present the case that MRgRT has the potential to become a widely utilized treatment platform and transform the radiation oncology treatment process just as earlier disruptive radiation therapy technologies have done.
Collapse
Affiliation(s)
- John Ng
- Department of Radiation Oncology, Weill Cornell Medicine, New York, NY, United States,*Correspondence: John Ng,
| | - Fabiana Gregucci
- Department of Radiation Oncology, Weill Cornell Medicine, New York, NY, United States,Department of Radiation Oncology, Miulli General Regional Hospital, Acquaviva delle Fonti, Bari, Italy
| | - Ryan T. Pennell
- Department of Radiation Oncology, Weill Cornell Medicine, New York, NY, United States
| | - Himanshu Nagar
- Department of Radiation Oncology, Weill Cornell Medicine, New York, NY, United States
| | - Encouse B. Golden
- Department of Radiation Oncology, Weill Cornell Medicine, New York, NY, United States
| | | | | | - Silvia C. Formenti
- Department of Radiation Oncology, Weill Cornell Medicine, New York, NY, United States
| |
Collapse
|
|
5
|
Wang XY, Beeraka NM, Xue NN, Yu HM, Yang Y, Liu MX, Nikolenko VN, Liu JQ, Zhao D. Identification of a three-gene prognostic signature for radioresistant esophageal squamous cell carcinoma. World J Clin Oncol 2023; 14:13-26. [PMID: 36699628 PMCID: PMC9850665 DOI: 10.5306/wjco.v14.i1.13] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 07/25/2022] [Revised: 10/25/2022] [Accepted: 12/06/2022] [Indexed: 01/10/2023] Open
Abstract
BACKGROUND Esophageal squamous cell carcinoma (ESCC) is causing a high mortality rate due to the lack of efficient early prognosis markers and suitable therapeutic regimens. The prognostic role of genes responsible for the acquisition of radioresistance in ESCC has not been fully elucidated.
AIM To establish a prognostic model by studying gene expression patterns pertinent to radioresistance in ESCC patients.
METHODS Datasets were obtained from the Gene Expression Omnibus and The Cancer Genome Atlas databases. The edgeR, a Bioconductor package, was used to analyze mRNA expression between different groups. We screened genes specifically responsible for radioresistance to estimate overall survival. Pearson correlation analysis was performed to confirm whether the expression of those genes correlated with each other. Genes contributing to radioresistance and overall survival were assessed by the multivariate Cox regression model through the calculation of βi and risk score using the following formula: 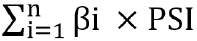 . .
RESULTS We identified three prognostic mRNAs (cathepsin S [CTSS], cluster of differentiation 180 [CD180], and SLP adapter and CSK-interacting membrane protein [SCIMP]) indicative of radioresistance. The expression of the three identified mRNAs was related to each other (r > 0.70 and P < 0.05). As to 1-year and 3-year overall survival prediction, the area under the time-dependent receiver operating characteristic curve of the signature consisting of the three mRNAs was 0.716 and 0.841, respectively. When stratifying patients based on the risk score derived from the signature, the high-risk group exhibited a higher death risk and shorter survival time than the low-risk group (P < 0.0001). Overall survival of the low-risk patients was significantly better than that of the high-risk patients (P = 0.018).
CONCLUSION We have developed a novel three-gene prognostic signature consisting of CTSS, CD180, and SCIMO for ESCC, which may facilitate the prediction of early prognosis of this malignancy.
Collapse
Affiliation(s)
- Xiao-Yan Wang
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Narasimha M Beeraka
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- Department of Pharmaceutical Chemistry, JSS College of Pharmacy, Mysuru 570015, India
| | - Nan-Nan Xue
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Hui-Ming Yu
- Department of Radiation Oncology, Peking University Cancer Hospital & Institute, Beijing 065005, China
| | - Ya Yang
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Mao-Xing Liu
- Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Gastrointestinal Surgery IV, Peking University Cancer Hospital & Institute, Beijing, China
| | - Vladimir N Nikolenko
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- M.V. Lomonosov Moscow State University, Moscow 119991, Russia
| | - Jun-Qi Liu
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Di Zhao
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| |
Collapse
|
|
6
|
Dhawan A, Pifer PM, Sandulache VC, Skinner HD. Metabolic targeting, immunotherapy and radiation in locally advanced non-small cell lung cancer: Where do we go from here? Front Oncol 2022; 12:1016217. [PMID: 36591457 PMCID: PMC9794617 DOI: 10.3389/fonc.2022.1016217] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/10/2022] [Accepted: 11/24/2022] [Indexed: 12/15/2022] Open
Abstract
In the US, there are ~250,000 new lung cancer diagnoses and ~130,000 deaths per year, and worldwide there are an estimated 1.6 million deaths per year from this deadly disease. Lung cancer is the most common cause of cancer death worldwide, and it accounts for roughly a quarter of all cancer deaths in the US. Non-small cell lung cancer (NSCLC) represents 80-85% of these cases. Due to an enormous tobacco cessation effort, NSCLC rates in the US are decreasing, and the implementation of lung cancer screening guidelines and other programs have resulted in a higher percentage of patients presenting with potentially curable locoregional disease, instead of distant disease. Exciting developments in molecular targeted therapy and immunotherapy have resulted in dramatic improvement in patients' survival, in combination with new surgical, pathological, radiographical, and radiation techniques. Concurrent platinum-based doublet chemoradiation therapy followed by immunotherapy has set the benchmark for survival in these patients. However, despite these advances, ~50% of patients diagnosed with locally advanced NSCLC (LA-NSCLC) survive long-term. In patients with local and/or locoregional disease, chemoradiation is a critical component of curative therapy. However, there remains a significant clinical gap in improving the efficacy of this combined therapy, and the development of non-overlapping treatment approaches to improve treatment outcomes is needed. One potential promising avenue of research is targeting cancer metabolism. In this review, we will initially provide a brief general overview of tumor metabolism as it relates to therapeutic targeting. We will then focus on the intersection of metabolism on both oxidative stress and anti-tumor immunity. This will be followed by discussion of both tumor- and patient-specific opportunities for metabolic targeting in NSCLC. We will then conclude with a discussion of additional agents currently in development that may be advantageous to combine with chemo-immuno-radiation in NSCLC.
Collapse
Affiliation(s)
- Annika Dhawan
- Department of Radiation Oncology, UPMC Hillman Cancer Center and University of Pittsburgh, Pittsburgh, PA, United States
| | - Phillip M. Pifer
- Department of Radiation Oncology, UPMC Hillman Cancer Center and University of Pittsburgh, Pittsburgh, PA, United States
| | - Vlad C. Sandulache
- Bobby R. Alford Department of Otolaryngology-Head and Neck Surgery, Baylor College of Medicine, Houston, TX, United States
| | - Heath D. Skinner
- Department of Radiation Oncology, UPMC Hillman Cancer Center and University of Pittsburgh, Pittsburgh, PA, United States,*Correspondence: Heath D. Skinner,
| |
Collapse
|
|
7
|
Enomoto A, Ichikawa K. Research and Development of Preclinical Overhauser-Enhanced Magnetic Resonance Imaging. Antioxid Redox Signal 2022; 37:1094-1110. [PMID: 35369734 DOI: 10.1089/ars.2022.0038] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Indexed: 11/12/2022]
Abstract
Significance: Imaging free radicals, including reactive oxygen species and reactive nitrogen species, can be useful for understanding the pathology of diseases in animal disease models, as they are related to various physiological functions or diseases. Among the methods used for imaging free radicals, Overhauser-enhanced magnetic resonance imaging (OMRI) has a short image acquisition time and high spatial resolution. Therefore, OMRI is used to obtain various biological parameters. In this study, we review the methodology for improving the biological OMRI system and its applications. Recent Advances: The sensitivity of OMRI systems has been enhanced significantly to allow the visualization of various biological parameters, such as redox state, partial oxygen pressure, and pH, in different body parts of small animals, using spin probes. Furthermore, both endogenous free radicals and exogenous free radicals present in drugs can be visualized using OMRI. Critical Issues: To acquire accurate biological parameters at a high resolution, it is essential to increase the electron paramagnetic resonance (EPR) excitation efficiency and achieve a high enhancement factor. In addition, the size and magnetic field strength also need to be optimized for the measurement target. Future Directions: The advancement of in vivo OMRI techniques will be useful for understanding the pathology, diagnosis, and evaluation of therapeutic effects of drugs in various disease models. Antioxid. Redox Signal. 37, 1094-1110.
Collapse
Affiliation(s)
- Ayano Enomoto
- Department of Biophysical Chemistry, Faculty of Pharmaceutical Sciences, Nagasaki International University, Sasebo, Japan
| | - Kazuhiro Ichikawa
- Department of Biophysical Chemistry, Faculty of Pharmaceutical Sciences, Nagasaki International University, Sasebo, Japan
| |
Collapse
|
|
8
|
Gurney-Champion OJ, Landry G, Redalen KR, Thorwarth D. Potential of Deep Learning in Quantitative Magnetic Resonance Imaging for Personalized Radiotherapy. Semin Radiat Oncol 2022; 32:377-388. [DOI: 10.1016/j.semradonc.2022.06.007] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 11/13/2022]
|
|
9
|
Read GH, Bailleul J, Vlashi E, Kesarwala AH. Metabolic response to radiation therapy in cancer. Mol Carcinog 2022; 61:200-224. [PMID: 34961986 PMCID: PMC10187995 DOI: 10.1002/mc.23379] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/11/2021] [Revised: 12/01/2021] [Accepted: 12/01/2021] [Indexed: 11/11/2022]
Abstract
Tumor metabolism has emerged as a hallmark of cancer and is involved in carcinogenesis and tumor growth. Reprogramming of tumor metabolism is necessary for cancer cells to sustain high proliferation rates and enhanced demands for nutrients. Recent studies suggest that metabolic plasticity in cancer cells can decrease the efficacy of anticancer therapies by enhancing antioxidant defenses and DNA repair mechanisms. Studying radiation-induced metabolic changes will lead to a better understanding of radiation response mechanisms as well as the identification of new therapeutic targets, but there are few robust studies characterizing the metabolic changes induced by radiation therapy in cancer. In this review, we will highlight studies that provide information on the metabolic changes induced by radiation and oxidative stress in cancer cells and the associated underlying mechanisms.
Collapse
Affiliation(s)
- Graham H. Read
- Department of Radiation Oncology, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California
| | - Justine Bailleul
- Department of Radiation Oncology, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California
| | - Erina Vlashi
- Department of Radiation Oncology, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California
- Jonsson Comprehensive Cancer Center, University of California, Los Angeles, Los Angeles, California
| | - Aparna H. Kesarwala
- Department of Radiation Oncology, Winship Cancer Institute, Emory University School of Medicine, Atlanta, Georgia
| |
Collapse
|
|
10
|
Yuan J, Poon DMC, Lo G, Wong OL, Cheung KY, Yu SK. A narrative review of MRI acquisition for MR-guided-radiotherapy in prostate cancer. Quant Imaging Med Surg 2022; 12:1585-1607. [PMID: 35111651 PMCID: PMC8739116 DOI: 10.21037/qims-21-697] [Citation(s) in RCA: 14] [Impact Index Per Article: 7.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/07/2021] [Accepted: 08/20/2021] [Indexed: 08/24/2023]
Abstract
Magnetic resonance guided radiotherapy (MRgRT), enabled by the clinical introduction of the integrated MRI and linear accelerator (MR-LINAC), is a novel technique for prostate cancer (PCa) treatment, promising to further improve clinical outcome and reduce toxicity. The role of prostate MRI has been greatly expanded from the traditional PCa diagnosis to also PCa screening, treatment and surveillance. Diagnostic prostate MRI has been relatively familiar in the community, particularly with the development of Prostate Imaging - Reporting and Data System (PI-RADS). But, on the other hand, the use of MRI in the emerging clinical practice of PCa MRgRT, which is substantially different from that in PCa diagnosis, has been so far sparsely presented in the medical literature. This review attempts to give a comprehensive overview of MRI acquisition techniques currently used in the clinical workflows of PCa MRgRT, from treatment planning to online treatment guidance, in order to promote MRI practice and research for PCa MRgRT. In particular, the major differences in the MRI acquisition of PCa MRgRT from that of diagnostic prostate MRI are demonstrated and explained. Limitations in the current MRI acquisition for PCa MRgRT are analyzed. The future developments of MRI in the PCa MRgRT are also discussed.
Collapse
Affiliation(s)
- Jing Yuan
- Medical Physics and Research Department, Hong Kong Sanatorium & Hospital, Hong Kong, China
| | - Darren M. C. Poon
- Comprehensive Oncology Centre, Hong Kong Sanatorium & Hospital, Hong Kong, China
| | - Gladys Lo
- Department of Diagnostic & Interventional Radiology, Hong Kong Sanatorium & Hospital, Hong Kong, China
| | - Oi Lei Wong
- Medical Physics and Research Department, Hong Kong Sanatorium & Hospital, Hong Kong, China
| | - Kin Yin Cheung
- Medical Physics and Research Department, Hong Kong Sanatorium & Hospital, Hong Kong, China
| | - Siu Ki Yu
- Medical Physics and Research Department, Hong Kong Sanatorium & Hospital, Hong Kong, China
| |
Collapse
|
|
11
|
Hwang I, Choi SH, Kim JW, Yeon EK, Lee JY, Yoo RE, Kang KM, Yun TJ, Kim JH, Sohn CH. Response prediction of vestibular schwannoma after gamma-knife radiosurgery using pretreatment dynamic contrast-enhanced MRI: a prospective study. Eur Radiol 2022; 32:3734-3743. [PMID: 35084518 DOI: 10.1007/s00330-021-08517-1] [Citation(s) in RCA: 2] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/07/2021] [Revised: 11/09/2021] [Accepted: 12/10/2021] [Indexed: 11/04/2022]
Abstract
OBJECTIVES There are few known predictive factors for response to gamma-knife radiosurgery (GKRS) in vestibular schwannoma (VS). We investigated the predictive role of pretreatment dynamic contrast-enhanced (DCE)-MRI parameters regarding the tumor response after GKRS in sporadic VS. METHODS This single-center prospective study enrolled participants between April 2017 and February 2019. We performed a volumetric measurement of DCE-MRI-derived parameters before GKRS. The tumor volume was measured in a follow-up MRI. The pharmacokinetic parameters were compared between responders and nonresponders according to 20% or more tumor volume reduction. Stepwise multivariable logistic regression analyses were performed, and the diagnostic performance of DCE-MRI parameters for the prediction of tumor response was evaluated by receiver operating characteristic curve analysis. RESULTS Ultimately, 35 participants (21 women, 52 ± 12 years) were included. There were 22 (62.9%) responders with a mean follow-up interval of 30.2 ± 5.7 months. Ktrans (0.036 min-1 vs. 0.057 min-1, p = .008) and initial area under the time-concentration curve within 90 s (IAUC90) (84.4 vs. 143.6, p = .003) showed significant differences between responders and nonresponders. Ktrans (OR = 0.96, p = .021) and IAUC90 (OR = 0.97, p = .004) were significant differentiating variables in each multivariable model with clinical variables for tumor response prediction. Ktrans showed a sensitivity of 81.8% and a specificity of 69.2%, and IAUC90 showed a sensitivity of 100% and a specificity of 53.8% for tumor response prediction. CONCLUSION DCE-MRI (particularly Ktrans and IAUC90) has the potential to be a predictive factor for tumor response in VS after GKRS. KEY POINTS •Pretreatment prediction of gamma-knife radiosurgery response in vestibular schwannoma is still challenging. •Dynamic contrast-enhanced MRI could have predictive value for the response of vestibular schwannoma after gamma-knife radiosurgery.
Collapse
Affiliation(s)
- Inpyeong Hwang
- Department of Radiology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea
| | - Seung Hong Choi
- Department of Radiology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea. .,Department of Radiology, Seoul National University College of Medicine, Seoul, Republic of Korea. .,Institute of Radiation Medicine, Seoul National University Medical Research Center, 103 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea. .,Center for Nanoparticle Research, Institute for Basic Science (IBS), Seoul, 08826, Republic of Korea.
| | - Jin Wook Kim
- Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Republic of Korea
| | - Eung Koo Yeon
- Department of Radiology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea
| | - Ji Ye Lee
- Department of Radiology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea
| | - Roh-Eul Yoo
- Department of Radiology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea.,Department of Radiology, Seoul National University College of Medicine, Seoul, Republic of Korea.,Institute of Radiation Medicine, Seoul National University Medical Research Center, 103 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea
| | - Koung Mi Kang
- Department of Radiology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea.,Department of Radiology, Seoul National University College of Medicine, Seoul, Republic of Korea
| | - Tae Jin Yun
- Department of Radiology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea.,Department of Radiology, Seoul National University College of Medicine, Seoul, Republic of Korea
| | - Ji-Hoon Kim
- Department of Radiology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea.,Department of Radiology, Seoul National University College of Medicine, Seoul, Republic of Korea
| | - Chul-Ho Sohn
- Department of Radiology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea.,Department of Radiology, Seoul National University College of Medicine, Seoul, Republic of Korea.,Institute of Radiation Medicine, Seoul National University Medical Research Center, 103 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea
| |
Collapse
|
|
12
|
Lallemand F, Leroi N, Blacher S, Bahri MA, Balteau E, Coucke P, Noël A, Plenevaux A, Martinive P. Tumor Microenvironment Modifications Recorded With IVIM Perfusion Analysis and DCE-MRI After Neoadjuvant Radiotherapy: A Preclinical Study. Front Oncol 2021; 11:784437. [PMID: 34993143 PMCID: PMC8724034 DOI: 10.3389/fonc.2021.784437] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/27/2021] [Accepted: 12/02/2021] [Indexed: 11/13/2022] Open
Abstract
PURPOSE Neoadjuvant radiotherapy (NeoRT) improves tumor local control and facilitates tumor resection in many cancers. Some clinical studies demonstrated that both timing of surgery and RT schedule influence tumor dissemination, and subsequently patient overall survival. Previously, we developed a pre-clinical model demonstrating the impact of NeoRT schedule and timing of surgery on metastatic spreading. We report on the impact of NeoRT on tumor microenvironment by MRI. METHODS According to our NeoRT model, MDA-MB 231 cells were implanted in the flank of SCID mice. Tumors were locally irradiated (PXI X-Rad SmART) with 2x5Gy and then surgically removed at different time points after RT. Diffusion-weighted (DW) and Dynamic contrast enhancement (DCE) MRI images were acquired before RT and every 2 days between RT and surgery. IntraVoxel Incoherent Motion (IVIM) analysis was used to obtain information on intravascular diffusion, related to perfusion (F: perfusion factor) and subsequently tumor vessels perfusion. For DCE-MRI, we performed semi-quantitative analyses. RESULTS With this experimental model, a significant and transient increase of the perfusion factor F [50% of the basal value (n=16, p<0.005)] was observed on day 6 after irradiation as well as a significant increase of the WashinSlope with DCE-MRI at day 6 (n=13, p<0.05). Using immunohistochemistry, a significant increase of perfused vessels was highlighted, corresponding to the increase of perfusion in MRI at this same time point. Moreover, Tumor surgical resection during this peak of vascularization results in an increase of metastasis burden (n=10, p<0.05). CONCLUSION Significant differences in perfusion-related parameters (F and WashinSlope) were observed on day 6 in a neoadjuvant radiotherapy model using SCID mice. These modifications are correlated with an increase of perfused vessels in histological analysis and also with an increase of metastasis spreading after the surgical procedure. This experimental observation could potentially result in a way to personalize treatment, by modulating the time of surgery guided on MRI functional data, especially tumor perfusion.
Collapse
Affiliation(s)
- François Lallemand
- Department of Radiotherapy-Oncology, Centre Hospitalier Universitaire (CHU) de Liège, University of Liège (ULg), Liège, Belgium
- Laboratory of Tumor and Development Biology, University of Liège (ULg), Liège, Belgium
- GIGA-Cyclotron Research Centre-in vivo Imaging, University of Liège, Liège, Belgium
| | - Natacha Leroi
- Laboratory of Tumor and Development Biology, University of Liège (ULg), Liège, Belgium
| | - Silvia Blacher
- Laboratory of Tumor and Development Biology, University of Liège (ULg), Liège, Belgium
| | - Mohamed Ali Bahri
- GIGA-Cyclotron Research Centre-in vivo Imaging, University of Liège, Liège, Belgium
| | - Evelyne Balteau
- GIGA-Cyclotron Research Centre-in vivo Imaging, University of Liège, Liège, Belgium
| | - Philippe Coucke
- Department of Radiotherapy-Oncology, Centre Hospitalier Universitaire (CHU) de Liège, University of Liège (ULg), Liège, Belgium
| | - Agnès Noël
- Laboratory of Tumor and Development Biology, University of Liège (ULg), Liège, Belgium
| | - Alain Plenevaux
- GIGA-Cyclotron Research Centre-in vivo Imaging, University of Liège, Liège, Belgium
| | - Philippe Martinive
- Laboratory of Tumor and Development Biology, University of Liège (ULg), Liège, Belgium
- Department of Radiotherapy-Oncology, Institut Jules Bordet, Université Libre de Bruxelles (ULB), Brussels, Belgium
| |
Collapse
|
|
13
|
Qin H, Tang S, Riselli AM, Bok RA, Delos Santos R, van Criekinge M, Gordon JW, Aggarwal R, Chen R, Goddard G, Zhang CT, Chen A, Reed G, Ruscitto DM, Slater J, Sriram R, Larson PEZ, Vigneron DB, Kurhanewicz J. Clinical translation of hyperpolarized 13 C pyruvate and urea MRI for simultaneous metabolic and perfusion imaging. Magn Reson Med 2021; 87:138-149. [PMID: 34374471 PMCID: PMC8616838 DOI: 10.1002/mrm.28965] [Citation(s) in RCA: 20] [Impact Index Per Article: 6.7] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 04/28/2021] [Revised: 06/30/2021] [Accepted: 07/23/2021] [Indexed: 11/11/2022]
Abstract
Purpose The combined hyperpolarized (HP) 13C pyruvate and urea MRI has provided a simultaneous assessment of glycolytic metabolism and tissue perfusion for improved cancer diagnosis and therapeutic evaluation in preclinical studies. This work aims to translate this dual‐probe HP imaging technique to clinical research. Methods A co‐polarization system was developed where [1‐13C]pyruvic acid (PA) and [13C, 15N2]urea in water solution were homogeneously mixed and polarized on a 5T SPINlab system. Physical and chemical characterizations and toxicology studies of the combined probe were performed. Simultaneous metabolic and perfusion imaging was performed on a 3T clinical MR scanner by alternatively applying a multi‐slice 2D spiral sequence for [1‐13C]pyruvate and its downstream metabolites and a 3D balanced steady‐state free precession (bSSFP) sequence for [13C, 15N2]urea. Results The combined PA/urea probe has a glass‐formation ability similar to neat PA and can generate nearly 40% liquid‐state 13C polarization for both pyruvate and urea in 3‐4 h. A standard operating procedure for routine on‐site production was developed and validated to produce 40 mL injection product of approximately 150 mM pyruvate and 35 mM urea. The toxicology study demonstrated the safety profile of the combined probe. Dynamic metabolite‐specific imaging of [1‐13C]pyruvate, [1‐13C]lactate, [1‐13C]alanine, and [13C, 15N2]urea was achieved with adequate spatial (2.6 mm × 2.6 mm) and temporal resolution (4.2 s), and urea images showed reduced off‐resonance artifacts due to the JCN coupling. Conclusion The reported technical development and translational studies will lead to the first‐in‐human dual‐agent HP MRI study and mark the clinical translation of the first HP 13C MRI probe after pyruvate.
Collapse
Affiliation(s)
- Hecong Qin
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA.,Graduate Program in Bioengineering, University of California, Berkeley and San Francisco, San Francisco, California, USA
| | - Shuyu Tang
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
| | - Andrew M Riselli
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
| | - Robert A Bok
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
| | - Romelyn Delos Santos
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
| | - Mark van Criekinge
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
| | - Jeremy W Gordon
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
| | - Rahul Aggarwal
- Department of Medicine, University of California, San Francisco, San Francisco, California, USA
| | - Rui Chen
- General Electric Healthcare, Milwaukee, Wisconsin, USA
| | | | | | - Albert Chen
- General Electric Healthcare, Milwaukee, Wisconsin, USA
| | - Galen Reed
- General Electric Healthcare, Milwaukee, Wisconsin, USA
| | | | - James Slater
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
| | - Renuka Sriram
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA
| | - Peder E Z Larson
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA.,Graduate Program in Bioengineering, University of California, Berkeley and San Francisco, San Francisco, California, USA
| | - Daniel B Vigneron
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA.,Graduate Program in Bioengineering, University of California, Berkeley and San Francisco, San Francisco, California, USA
| | - John Kurhanewicz
- Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA.,Graduate Program in Bioengineering, University of California, Berkeley and San Francisco, San Francisco, California, USA
| |
Collapse
|
|
14
|
Bridging cell-scale simulations and radiologic images to explain short-time intratumoral oxygen fluctuations. PLoS Comput Biol 2021; 17:e1009206. [PMID: 34310608 PMCID: PMC8341701 DOI: 10.1371/journal.pcbi.1009206] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/04/2021] [Revised: 08/05/2021] [Accepted: 06/22/2021] [Indexed: 11/19/2022] Open
Abstract
Radiologic images provide a way to monitor tumor development and its response to therapies in a longitudinal and minimally invasive fashion. However, they operate on a macroscopic scale (average value per voxel) and are not able to capture microscopic scale (cell-level) phenomena. Nevertheless, to examine the causes of frequent fast fluctuations in tissue oxygenation, models simulating individual cells’ behavior are needed. Here, we provide a link between the average data values recorded for radiologic images and the cellular and vascular architecture of the corresponding tissues. Using hybrid agent-based modeling, we generate a set of tissue morphologies capable of reproducing oxygenation levels observed in radiologic images. We then use these in silico tissues to investigate whether oxygen fluctuations can be explained by changes in vascular oxygen supply or by modulations in cellular oxygen absorption. Our studies show that intravascular changes in oxygen supply reproduce the observed fluctuations in tissue oxygenation in all considered regions of interest. However, larger-magnitude fluctuations cannot be recreated by modifications in cellular absorption of oxygen in a biologically feasible manner. Additionally, we develop a procedure to identify plausible tissue morphologies for a given temporal series of average data from radiology images. In future applications, this approach can be used to generate a set of tissues comparable with radiology images and to simulate tumor responses to various anti-cancer treatments at the tissue-scale level. Low levels of oxygen, called hypoxia, are observable in many solid tumors. They are associated with more aggressive malignant cells that are resistant to chemo-, radio-, and immunotherapies. Recently developed imaging techniques provide a way to measure the magnitude of frequent short-term oxygen fluctuations, but they operate on a macro-scale voxel level. To examine the possible causes of rapid oxygen fluctuations at the cell level, we developed a hybrid agent-based mathematical model. We tested two different mechanisms that may be responsible for these cyclic effects on tissue oxygenation: temporal variations in vascular influx of oxygen and modulations in cellular oxygen absorption. Additionally, we developed a procedure to identify plausible tissue morphologies from data collected from radiological images. This can provide a bridge between the micro-scale simulations with individual cells and the longitudinal medical images containing average values. In future applications, this approach can be used to generate a set of tissues compatible with radiology images and to simulate tumor responses to various anticancer treatments at the cell-scale level.
Collapse
|
|
15
|
Gröhl J, Schellenberg M, Dreher K, Maier-Hein L. Deep learning for biomedical photoacoustic imaging: A review. PHOTOACOUSTICS 2021; 22:100241. [PMID: 33717977 PMCID: PMC7932894 DOI: 10.1016/j.pacs.2021.100241] [Citation(s) in RCA: 100] [Impact Index Per Article: 33.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Subscribe] [Scholar Register] [Received: 11/05/2020] [Revised: 01/18/2021] [Accepted: 01/20/2021] [Indexed: 05/04/2023]
Abstract
Photoacoustic imaging (PAI) is a promising emerging imaging modality that enables spatially resolved imaging of optical tissue properties up to several centimeters deep in tissue, creating the potential for numerous exciting clinical applications. However, extraction of relevant tissue parameters from the raw data requires the solving of inverse image reconstruction problems, which have proven extremely difficult to solve. The application of deep learning methods has recently exploded in popularity, leading to impressive successes in the context of medical imaging and also finding first use in the field of PAI. Deep learning methods possess unique advantages that can facilitate the clinical translation of PAI, such as extremely fast computation times and the fact that they can be adapted to any given problem. In this review, we examine the current state of the art regarding deep learning in PAI and identify potential directions of research that will help to reach the goal of clinical applicability.
Collapse
Affiliation(s)
- Janek Gröhl
- German Cancer Research Center, Computer Assisted Medical Interventions, Heidelberg, Germany
- Heidelberg University, Medical Faculty, Heidelberg, Germany
| | - Melanie Schellenberg
- German Cancer Research Center, Computer Assisted Medical Interventions, Heidelberg, Germany
| | - Kris Dreher
- German Cancer Research Center, Computer Assisted Medical Interventions, Heidelberg, Germany
- Heidelberg University, Faculty of Physics and Astronomy, Heidelberg, Germany
| | - Lena Maier-Hein
- German Cancer Research Center, Computer Assisted Medical Interventions, Heidelberg, Germany
- Heidelberg University, Medical Faculty, Heidelberg, Germany
- Heidelberg University, Faculty of Mathematics and Computer Science, Heidelberg, Germany
| |
Collapse
|
|
16
|
McGee KP, Hwang KP, Sullivan DC, Kurhanewicz J, Hu Y, Wang J, Li W, Debbins J, Paulson E, Olsen JR, Hua CH, Warner L, Ma D, Moros E, Tyagi N, Chung C. Magnetic resonance biomarkers in radiation oncology: The report of AAPM Task Group 294. Med Phys 2021; 48:e697-e732. [PMID: 33864283 PMCID: PMC8361924 DOI: 10.1002/mp.14884] [Citation(s) in RCA: 12] [Impact Index Per Article: 4.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/19/2020] [Revised: 03/24/2021] [Accepted: 03/28/2021] [Indexed: 12/16/2022] Open
Abstract
A magnetic resonance (MR) biologic marker (biomarker) is a measurable quantitative characteristic that is an indicator of normal biological and pathogenetic processes or a response to therapeutic intervention derived from the MR imaging process. There is significant potential for MR biomarkers to facilitate personalized approaches to cancer care through more precise disease targeting by quantifying normal versus pathologic tissue function as well as toxicity to both radiation and chemotherapy. Both of which have the potential to increase the therapeutic ratio and provide earlier, more accurate monitoring of treatment response. The ongoing integration of MR into routine clinical radiation therapy (RT) planning and the development of MR guided radiation therapy systems is providing new opportunities for MR biomarkers to personalize and improve clinical outcomes. Their appropriate use, however, must be based on knowledge of the physical origin of the biomarker signal, the relationship to the underlying biological processes, and their strengths and limitations. The purpose of this report is to provide an educational resource describing MR biomarkers, the techniques used to quantify them, their strengths and weakness within the context of their application to radiation oncology so as to ensure their appropriate use and application within this field.
Collapse
Affiliation(s)
- Kiaran P McGee
- Department of Radiology, Mayo Clinic, Rochester, Minnesota, USA
| | - Ken-Pin Hwang
- Department of Imaging Physics, Division of Diagnostic Imaging, MD Anderson Cancer Center, University of Texas, Houston, Texas, USA
| | - Daniel C Sullivan
- Department of Radiology, Duke University, Durham, North Carolina, USA
| | - John Kurhanewicz
- Department of Radiology, University of California, San Francisco, California, USA
| | - Yanle Hu
- Department of Radiation Oncology, Mayo Clinic, Scottsdale, Arizona, USA
| | - Jihong Wang
- Department of Radiation Oncology, MD Anderson Cancer Center, University of Texas, Houston, Texas, USA
| | - Wen Li
- Department of Radiation Oncology, University of Arizona, Tucson, Arizona, USA
| | - Josef Debbins
- Department of Radiology, Barrow Neurologic Institute, Phoenix, Arizona, USA
| | - Eric Paulson
- Department of Radiation Oncology, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
| | - Jeffrey R Olsen
- Department of Radiation Oncology, University of Colorado Denver - Anschutz Medical Campus, Denver, Colorado, USA
| | - Chia-Ho Hua
- Department of Radiation Oncology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA
| | | | - Daniel Ma
- Department of Radiation Oncology, Mayo Clinic, Rochester, Minnesota, USA
| | - Eduardo Moros
- Department of Radiation Oncology, Moffitt Cancer Center, Tampa, Florida, USA
| | - Neelam Tyagi
- Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, New York, USA
| | - Caroline Chung
- Department of Radiation Oncology, MD Anderson Cancer Center, University of Texas, Houston, Texas, USA
| |
Collapse
|
|
17
|
D'Alonzo RA, Gill S, Rowshanfarzad P, Keam S, MacKinnon KM, Cook AM, Ebert MA. In vivo noninvasive preclinical tumor hypoxia imaging methods: a review. Int J Radiat Biol 2021; 97:593-631. [PMID: 33703994 DOI: 10.1080/09553002.2021.1900943] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/16/2020] [Revised: 01/28/2021] [Accepted: 03/01/2021] [Indexed: 12/15/2022]
Abstract
Tumors exhibit areas of decreased oxygenation due to malformed blood vessels. This low oxygen concentration decreases the effectiveness of radiation therapy, and the resulting poor perfusion can prevent drugs from reaching areas of the tumor. Tumor hypoxia is associated with poorer prognosis and disease progression, and is therefore of interest to preclinical researchers. Although there are multiple different ways to measure tumor hypoxia and related factors, there is no standard for quantifying spatial and temporal tumor hypoxia distributions in preclinical research or in the clinic. This review compares imaging methods utilized for the purpose of assessing spatio-temporal patterns of hypoxia in the preclinical setting. Imaging methods provide varying levels of spatial and temporal resolution regarding different aspects of hypoxia, and with varying advantages and disadvantages. The choice of modality requires consideration of the specific experimental model, the nature of the required characterization and the availability of complementary modalities as well as immunohistochemistry.
Collapse
Affiliation(s)
- Rebecca A D'Alonzo
- School of Physics, Mathematics and Computing, The University of Western Australia, Crawley, Australia
| | - Suki Gill
- School of Physics, Mathematics and Computing, The University of Western Australia, Crawley, Australia
- Department of Radiation Oncology, Sir Charles Gairdner Hospital, Nedlands, Australia
| | - Pejman Rowshanfarzad
- School of Physics, Mathematics and Computing, The University of Western Australia, Crawley, Australia
| | - Synat Keam
- School of Medicine, The University of Western Australia, Crawley, Australia
| | - Kelly M MacKinnon
- School of Physics, Mathematics and Computing, The University of Western Australia, Crawley, Australia
| | - Alistair M Cook
- School of Medicine, The University of Western Australia, Crawley, Australia
| | - Martin A Ebert
- School of Physics, Mathematics and Computing, The University of Western Australia, Crawley, Australia
- Department of Radiation Oncology, Sir Charles Gairdner Hospital, Nedlands, Australia
- 5D Clinics, Claremont, Australia
| |
Collapse
|
|
18
|
Prasad S, Chandra A, Cavo M, Parasido E, Fricke S, Lee Y, D'Amone E, Gigli G, Albanese C, Rodriguez O, Del Mercato LL. Optical and magnetic resonance imaging approaches for investigating the tumour microenvironment: state-of-the-art review and future trends. NANOTECHNOLOGY 2021; 32:062001. [PMID: 33065554 DOI: 10.1088/1361-6528/abc208] [Citation(s) in RCA: 6] [Impact Index Per Article: 2.0] [Reference Citation Analysis] [Abstract] [MESH Headings] [Track Full Text] [Subscribe] [Scholar Register] [Indexed: 06/11/2023]
Abstract
The tumour microenvironment (TME) strongly influences tumorigenesis and metastasis. Two of the most characterized properties of the TME are acidosis and hypoxia, both of which are considered hallmarks of tumours as well as critical factors in response to anticancer treatments. Currently, various imaging approaches exist to measure acidosis and hypoxia in the TME, including magnetic resonance imaging (MRI), positron emission tomography and optical imaging. In this review, we will focus on the latest fluorescent-based methods for optical sensing of cell metabolism and MRI as diagnostic imaging tools applied both in vitro and in vivo. The primary emphasis will be on describing the current and future uses of systems that can measure intra- and extra-cellular pH and oxygen changes at high spatial and temporal resolution. In addition, the suitability of these approaches for mapping tumour heterogeneity, and assessing response or failure to therapeutics will also be covered.
Collapse
Affiliation(s)
- Saumya Prasad
- Institute of Nanotechnology, National Research Council (CNR-NANOTEC), c/o Campus Ecotekne, via Monteroni, 73100, Lecce, Italy
| | - Anil Chandra
- Institute of Nanotechnology, National Research Council (CNR-NANOTEC), c/o Campus Ecotekne, via Monteroni, 73100, Lecce, Italy
| | - Marta Cavo
- Institute of Nanotechnology, National Research Council (CNR-NANOTEC), c/o Campus Ecotekne, via Monteroni, 73100, Lecce, Italy
| | - Erika Parasido
- Department of Oncology, Georgetown University Medical Center, Washington, DC, United States of America
- Center for Translational Imaging, Georgetown University Medical Center, Washington, DC, United States of America
| | - Stanley Fricke
- Department of Oncology, Georgetown University Medical Center, Washington, DC, United States of America
- Center for Translational Imaging, Georgetown University Medical Center, Washington, DC, United States of America
- Department of Radiology, Georgetown University Medical Center, Washington, DC, United States of America
| | - Yichien Lee
- Department of Oncology, Georgetown University Medical Center, Washington, DC, United States of America
| | - Eliana D'Amone
- Institute of Nanotechnology, National Research Council (CNR-NANOTEC), c/o Campus Ecotekne, via Monteroni, 73100, Lecce, Italy
| | - Giuseppe Gigli
- Institute of Nanotechnology, National Research Council (CNR-NANOTEC), c/o Campus Ecotekne, via Monteroni, 73100, Lecce, Italy
- Department of Mathematics and Physics 'Ennio De Giorgi', University of Salento, via Arnesano, 73100, Lecce, Italy
| | - Chris Albanese
- Department of Oncology, Georgetown University Medical Center, Washington, DC, United States of America
- Center for Translational Imaging, Georgetown University Medical Center, Washington, DC, United States of America
- Department of Radiology, Georgetown University Medical Center, Washington, DC, United States of America
| | - Olga Rodriguez
- Department of Oncology, Georgetown University Medical Center, Washington, DC, United States of America
- Center for Translational Imaging, Georgetown University Medical Center, Washington, DC, United States of America
| | - Loretta L Del Mercato
- Institute of Nanotechnology, National Research Council (CNR-NANOTEC), c/o Campus Ecotekne, via Monteroni, 73100, Lecce, Italy
| |
Collapse
|
|
19
|
Anemone A, Consolino L, Conti L, Irrera P, Hsu MY, Villano D, Dastrù W, Porporato PE, Cavallo F, Longo DL. Tumour acidosis evaluated in vivo by MRI-CEST pH imaging reveals breast cancer metastatic potential. Br J Cancer 2021; 124:207-216. [PMID: 33257841 PMCID: PMC7782702 DOI: 10.1038/s41416-020-01173-0] [Citation(s) in RCA: 38] [Impact Index Per Article: 12.7] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/18/2019] [Revised: 10/07/2020] [Accepted: 10/28/2020] [Indexed: 02/07/2023] Open
Abstract
BACKGROUND Tumour acidosis is considered to play a central role in promoting cancer invasion and migration, but few studies have investigated in vivo how tumour pH correlates with cancer invasion. This study aims to determine in vivo whether tumour acidity is associated with cancer metastatic potential. METHODS Breast cancer cell lines with different metastatic potentials have been characterised for several markers of aggressiveness and invasiveness. Murine tumour models have been developed and assessed for lung metastases and tumour acidosis has been assessed in vivo by a magnetic resonance imaging-based chemical exchange saturation transfer (CEST) pH imaging approach. RESULTS The higher metastatic potential of 4T1 and TS/A primary tumours, in comparison to the less aggressive TUBO and BALB-neuT ones, was confirmed by the highest expression of cancer cell stem markers (CD44+CD24-), highlighting their propensity to migrate and invade, coinciding with the measurement obtained by in vitro assays. MRI-CEST pH imaging successfully discriminated the more aggressive 4T1 and TS/A tumours that displayed a more acidic pH. Moreover, the observed higher tumour acidity was significantly correlated with an increased number of lung metastases. CONCLUSIONS The findings of this study indicate that the extracellular acidification is associated with the metastatic potential.
Collapse
Affiliation(s)
- Annasofia Anemone
- Department of Molecular Biotechnology and Health Sciences, Molecular Imaging Center, University of Torino, Via Nizza 52, Torino, Italy
| | - Lorena Consolino
- Department of Molecular Biotechnology and Health Sciences, Molecular Imaging Center, University of Torino, Via Nizza 52, Torino, Italy
| | - Laura Conti
- Department of Molecular Biotechnology and Health Sciences, University of Torino, Via Nizza 52, Torino, Italy
| | - Pietro Irrera
- University of Campania "Luigi Vanvitelli", Viale Abramo Lincoln, 5, Caserta, Italy
| | - Myriam Y Hsu
- Department of Molecular Biotechnology and Health Sciences, Molecular Imaging Center, University of Torino, Via Nizza 52, Torino, Italy
| | - Daisy Villano
- Department of Molecular Biotechnology and Health Sciences, Molecular Imaging Center, University of Torino, Via Nizza 52, Torino, Italy
| | - Walter Dastrù
- Department of Molecular Biotechnology and Health Sciences, Molecular Imaging Center, University of Torino, Via Nizza 52, Torino, Italy
| | - Paolo E Porporato
- Department of Molecular Biotechnology and Health Sciences, University of Torino, Via Nizza 52, Torino, Italy
| | - Federica Cavallo
- Department of Molecular Biotechnology and Health Sciences, University of Torino, Via Nizza 52, Torino, Italy
| | - Dario Livio Longo
- Institute of Biostructures and Bioimaging (IBB), Italian National Research Council (CNR), Via Nizza 52, Torino, Italy.
| |
Collapse
|
|
20
|
He Y, Mao Z, Zhang Y, Lv H, Yan J, Cao Y, Pei R. Tumor Acid Microenvironment-Triggered Self-Assembly of ESIONPs for T 1/T 2 Switchable Magnetic Resonance Imaging. ACS APPLIED BIO MATERIALS 2020; 3:7752-7761. [PMID: 35019515 DOI: 10.1021/acsabm.0c00958] [Citation(s) in RCA: 7] [Impact Index Per Article: 1.8] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 11/28/2022]
Abstract
Smart magnetic resonance imaging (MRI) contrast agents (CAs), whose MRI contrasting enhancement is variable in response to the specific stimulus from tumor tissues, possess great potential in precise tumor diagnosis. Herein, we design a type of extremely small iron oxide nanoparticle (ESIONP)-based pH-responsive system for activatable T2 MRI in the tumor acid microenvironment. The ESIONP system is composed of ESIONP-PEG-PGA and ESIONP-PEG-PDC, which were respectively constructed through the surface modification with poly (l-glutamic acid) (PGA) and poly(N-{N'-[N″-(2-carbox aminoethyl)]-2-aminoethyl}glutamide) (PDC) on the surface of ESIONP. The pH-responsive system exhibits the dispersed state under the neutral condition, and when it is exposed to the weakly acid environment, ESIONP-PEG-PDC switches from the neutral to positive charge, finally leading to the aggregation by the electrostatic interaction between the positively charged ESIONP-PEG-PDC and negatively charged ESIONP-PEG-PGA. On the basis of the aggregation, the T1 contrasting effect of the pH-responsive system switches to a T2 contrasting effect, which can be employed to realize the selective enhancement of imaging contrast at the tumor location owing to the weakly acid microenvironment. Moreover, on the basis of size increase originated from the aggregation effect, the residence time of extremely small iron oxide nanoparticles (ESIONPs) in the tumor site is effectively prolonged, which is beneficial for the MRI of tumors. Therefore, the pH-responsive system based on the ESIONPs is a potential smart MRI contrast agent for accurate tumor diagnosis.
Collapse
Affiliation(s)
- Yilin He
- CAS Key Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China.,Nano Science and Technology Institute, University of Science and Technology of China, Suzhou 215123, China
| | - Zheng Mao
- CAS Key Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China
| | - Ye Zhang
- CAS Key Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China
| | - Haiyin Lv
- CAS Key Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China
| | - Jincong Yan
- CAS Key Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China
| | - Yi Cao
- CAS Key Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China
| | - Renjun Pei
- CAS Key Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China
| |
Collapse
|
|
21
|
Circulating levels of hydroxylated bradykinin function as an indicator of tissue HIF-1α expression. Sci Bull (Beijing) 2020; 65:1570-1579. [PMID: 36738075 DOI: 10.1016/j.scib.2020.04.023] [Citation(s) in RCA: 3] [Impact Index Per Article: 0.8] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/26/2019] [Revised: 03/27/2020] [Accepted: 04/14/2020] [Indexed: 02/07/2023]
Abstract
The critical roles of oxygen homeostasis in metabolism are indisputable and hypoxic responses are correlated with the pathogenesis of gastrointestinal, pulmonary, renal diseases and cancers. Evaluating tissue hypoxia to predict treatment outcome is challenging, however, due to the lack of rapid, accurate and non-invasive methods. Hypoxia enhances prolyl-4-hydroxylase α1 (P4HA1) expression, which can convert bradykinin (BK) to hydroxyprolyl-BK (Hyp-BK), leading us to hypothesize that circulating Hyp-BK/BK ratios may reflect tissue hypoxia and predict treatment outcomes. Direct quantification of Hyp-BK peptides in serum or plasma by conventional MALDI-TOF MS analysis is technically challenging. In our study, a nanopore-based fractionation and enrichment protocol was utilized to allow the simple workflow for circulating Hyp-BK/BK analysis. Hypoxia is linked to poor prognosis due to its role in promoting pancreatic cancer progression and metastasis. Here we show that P4HA1 expression was increased in pancreatic tumors versus adjacent tissue, associated with poor survival, and corresponded with tumor expression of the hypoxia inducible factor 1α (HIF-1α) and carbonic anhydrase 9 (CA9). Hypoxia-induced P4HA1 expression and BK conversion to Hyp-BK were found to be HIF-1α dependent, pre-treatment serum Hyp-BK/BK ratios corresponded with tissue HIF-1α and P4HA1 expression, and high Hyp-BK/BK levels corresponded with poor response to therapy. These results suggest that pre-treatment circulating Hyp-BK/BK ratios may have value as a non-invasive, surrogate indicator of tissue hypoxia and tumor responses to therapy.
Collapse
|
|
22
|
Hu Q, Yu VY, Yang Y, Hu P, Sheng K, Lee PP, Kishan AU, Raldow AC, O'Connell DP, Woods KE, Cao M. Practical Safety Considerations for Integration of Magnetic Resonance Imaging in Radiation Therapy. Pract Radiat Oncol 2020; 10:443-453. [PMID: 32781246 DOI: 10.1016/j.prro.2020.07.008] [Citation(s) in RCA: 6] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/18/2020] [Revised: 07/16/2020] [Accepted: 07/28/2020] [Indexed: 12/29/2022]
Abstract
Interest in integrating magnetic resonance imaging (MRI) in radiation therapy (RT) practice has increased dramatically in recent years owing to its unique advantages such as excellent soft tissue contrast and capability of measuring biological properties. Continuous real-time imaging for intrafractional motion tracking without ionizing radiation serves as a particularly attractive feature for applications in RT. Despite its many advantages, the integration of MRI in RT workflows is not straightforward, with many unmet needs. MR safety remains one of the key challenges and concerns in the clinical implementation of MR simulators and MR-guided radiation therapy systems in radiation oncology. Most RT staff are not accustomed to working in an environment with a strong magnetic field. There are specific requirements in RT that are different from diagnostic applications. A large variety of implants and devices used in routine RT practice do not have clear MR safety labels. RT-specific imaging pulse sequences focusing on fast acquisition, high spatial integrity, and continuous, real-time acquisition require additional MR safety testing and evaluation. This article provides an overview of MR safety tailored toward RT staff, followed by discussions on specific requirements and challenges associated with MR safety in the RT environment. Strategies and techniques for developing an MR safety program specific to RT are presented and discussed.
Collapse
Affiliation(s)
- Qiongge Hu
- Department of Radiation Oncology, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
| | - Victoria Y Yu
- Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, New York
| | - Yingli Yang
- Department of Radiation Oncology, University of California, Los Angeles, California
| | - Peng Hu
- Department of Radiology, University of California, Los Angeles, California
| | - Ke Sheng
- Department of Radiation Oncology, University of California, Los Angeles, California
| | - Percy P Lee
- Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, Texas
| | - Amar U Kishan
- Department of Radiation Oncology, University of California, Los Angeles, California
| | - Ann C Raldow
- Department of Radiation Oncology, University of California, Los Angeles, California
| | - Dylan P O'Connell
- Department of Radiation Oncology, University of California, Los Angeles, California
| | - Kaley E Woods
- Department of Radiation Oncology, University of California, Los Angeles, California
| | - Minsong Cao
- Department of Radiation Oncology, University of California, Los Angeles, California.
| |
Collapse
|
|
23
|
Gurney-Champion OJ, Mahmood F, van Schie M, Julian R, George B, Philippens MEP, van der Heide UA, Thorwarth D, Redalen KR. Quantitative imaging for radiotherapy purposes. Radiother Oncol 2020; 146:66-75. [PMID: 32114268 PMCID: PMC7294225 DOI: 10.1016/j.radonc.2020.01.026] [Citation(s) in RCA: 61] [Impact Index Per Article: 15.3] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/19/2019] [Revised: 01/22/2020] [Accepted: 01/29/2020] [Indexed: 02/07/2023]
Abstract
Quantitative imaging biomarkers show great potential for use in radiotherapy. Quantitative images based on microscopic tissue properties and tissue function can be used to improve contouring of the radiotherapy targets. Furthermore, quantitative imaging biomarkers might be used to predict treatment response for several treatment regimens and hence be used as a tool for treatment stratification, either to determine which treatment modality is most promising or to determine patient-specific radiation dose. Finally, patient-specific radiation doses can be further tailored to a tissue/voxel specific radiation dose when quantitative imaging is used for dose painting. In this review, published standards, guidelines and recommendations on quantitative imaging assessment using CT, PET and MRI are discussed. Furthermore, critical issues regarding the use of quantitative imaging for radiation oncology purposes and resultant pending research topics are identified.
Collapse
Affiliation(s)
- Oliver J Gurney-Champion
- Joint Department of Physics, The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, London, United Kingdom.
| | - Faisal Mahmood
- Department of Oncology, Odense University Hospital, Denmark; Department of Clinical Research, University of Southern Denmark, Odense, Denmark
| | - Marcel van Schie
- Department of Radiation Oncology, the Netherlands Cancer Institute, Amsterdam, The Netherlands
| | - Robert Julian
- Department of Radiotherapy Physics, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom
| | - Ben George
- Radiation Therapy Medical Physics Group, CRUK/MRC Oxford Institute for Radiation Oncology, University of Oxford, United Kingdom
| | | | - Uulke A van der Heide
- Department of Radiation Oncology, the Netherlands Cancer Institute, Amsterdam, The Netherlands
| | - Daniela Thorwarth
- Section for Biomedical Physics, Department of Radiation Oncology, Eberhard Karls University of Tübingen, Germany
| | - Kathrine R Redalen
- Department of Physics, Norwegian University of Science and Technology, Trondheim, Norway
| |
Collapse
|
|
24
|
Consolino L, Anemone A, Capozza M, Carella A, Irrera P, Corrado A, Dhakan C, Bracesco M, Longo DL. Non-invasive Investigation of Tumor Metabolism and Acidosis by MRI-CEST Imaging. Front Oncol 2020; 10:161. [PMID: 32133295 PMCID: PMC7040491 DOI: 10.3389/fonc.2020.00161] [Citation(s) in RCA: 34] [Impact Index Per Article: 8.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/13/2019] [Accepted: 01/29/2020] [Indexed: 12/15/2022] Open
Abstract
Altered metabolism is considered a core hallmark of cancer. By monitoring in vivo metabolites changes or characterizing the tumor microenvironment, non-invasive imaging approaches play a fundamental role in elucidating several aspects of tumor biology. Within the magnetic resonance imaging (MRI) modality, the chemical exchange saturation transfer (CEST) approach has emerged as a new technique that provides high spatial resolution and sensitivity for in vivo imaging of tumor metabolism and acidosis. This mini-review describes CEST-based methods to non-invasively investigate tumor metabolism and important metabolites involved, such as glucose and lactate, as well as measurement of tumor acidosis. Approaches that have been exploited to assess response to anticancer therapies will also be reported for each specific technique.
Collapse
Affiliation(s)
- Lorena Consolino
- Department of Nanomedicines and Theranostics, Institute for Experimental Molecular Imaging, RWTH Aachen University, Aachen, Germany.,Department of Molecular Biotechnology and Health Sciences, Molecular Imaging Center, University of Torino, Turin, Italy
| | - Annasofia Anemone
- Department of Molecular Biotechnology and Health Sciences, Molecular Imaging Center, University of Torino, Turin, Italy
| | - Martina Capozza
- Department of Molecular Biotechnology and Health Sciences, Molecular Imaging Center, University of Torino, Turin, Italy
| | - Antonella Carella
- Institute of Biostructures and Bioimaging (IBB), Italian National Research Council (CNR), Turin, Italy
| | - Pietro Irrera
- University of Campania "Luigi Vanvitelli", Naples, Italy
| | - Alessia Corrado
- Institute of Biostructures and Bioimaging (IBB), Italian National Research Council (CNR), Turin, Italy
| | - Chetan Dhakan
- Institute of Biostructures and Bioimaging (IBB), Italian National Research Council (CNR), Turin, Italy.,University of Campania "Luigi Vanvitelli", Naples, Italy
| | - Martina Bracesco
- Department of Molecular Biotechnology and Health Sciences, Molecular Imaging Center, University of Torino, Turin, Italy
| | - Dario Livio Longo
- Institute of Biostructures and Bioimaging (IBB), Italian National Research Council (CNR), Turin, Italy
| |
Collapse
|
|
25
|
Ardaševa A, Gatenby RA, Anderson ARA, Byrne HM, Maini PK, Lorenzi T. Evolutionary dynamics of competing phenotype-structured populations in periodically fluctuating environments. J Math Biol 2020; 80:775-807. [PMID: 31641842 PMCID: PMC7028828 DOI: 10.1007/s00285-019-01441-5] [Citation(s) in RCA: 20] [Impact Index Per Article: 5.0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 05/08/2019] [Revised: 08/14/2019] [Indexed: 12/20/2022]
Abstract
Living species, ranging from bacteria to animals, exist in environmental conditions that exhibit spatial and temporal heterogeneity which requires them to adapt. Risk-spreading through spontaneous phenotypic variations is a known concept in ecology, which is used to explain how species may survive when faced with the evolutionary risks associated with temporally varying environments. In order to support a deeper understanding of the adaptive role of spontaneous phenotypic variations in fluctuating environments, we consider a system of non-local partial differential equations modelling the evolutionary dynamics of two competing phenotype-structured populations in the presence of periodically oscillating nutrient levels. The two populations undergo heritable, spontaneous phenotypic variations at different rates. The phenotypic state of each individual is represented by a continuous variable, and the phenotypic landscape of the populations evolves in time due to variations in the nutrient level. Exploiting the analytical tractability of our model, we study the long-time behaviour of the solutions to obtain a detailed mathematical depiction of the evolutionary dynamics. The results suggest that when nutrient levels undergo small and slow oscillations, it is evolutionarily more convenient to rarely undergo spontaneous phenotypic variations. Conversely, under relatively large and fast periodic oscillations in the nutrient levels, which bring about alternating cycles of starvation and nutrient abundance, higher rates of spontaneous phenotypic variations confer a competitive advantage. We discuss the implications of our results in the context of cancer metabolism.
Collapse
Affiliation(s)
- Aleksandra Ardaševa
- Wolfson Centre for Mathematical Biology, Mathematical Institute, University of Oxford, Andrew Wiles Building, Radcliffe Observatory Quarter, Woodstock Road, Oxford, OX2 6GG UK
| | - Robert A. Gatenby
- Department of Integrated Mathematical Oncology, H. Lee Moffitt Cancer Center, Tampa, FL USA
| | | | - Helen M. Byrne
- Wolfson Centre for Mathematical Biology, Mathematical Institute, University of Oxford, Andrew Wiles Building, Radcliffe Observatory Quarter, Woodstock Road, Oxford, OX2 6GG UK
| | - Philip K. Maini
- Wolfson Centre for Mathematical Biology, Mathematical Institute, University of Oxford, Andrew Wiles Building, Radcliffe Observatory Quarter, Woodstock Road, Oxford, OX2 6GG UK
| | - Tommaso Lorenzi
- School of Mathematics and Statistics, University of St Andrews, St Andrews, KY16 9SS UK
| |
Collapse
|
|
26
|
Chin S, Eccles CL, McWilliam A, Chuter R, Walker E, Whitehurst P, Berresford J, Van Herk M, Hoskin PJ, Choudhury A. Magnetic resonance-guided radiation therapy: A review. J Med Imaging Radiat Oncol 2020; 64:163-177. [PMID: 31646742 DOI: 10.1111/1754-9485.12968] [Citation(s) in RCA: 91] [Impact Index Per Article: 22.8] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/25/2019] [Accepted: 09/24/2019] [Indexed: 12/11/2022]
Abstract
Magnetic resonance-guided radiation therapy (MRgRT) is a promising approach to improving clinical outcomes for patients treated with radiation therapy. The roles of image guidance, adaptive planning and magnetic resonance imaging in radiation therapy have been increasing over the last two decades. Technical advances have led to the feasible combination of magnetic resonance imaging and radiation therapy technologies, leading to improved soft-tissue visualisation, assessment of inter- and intrafraction motion, motion management, online adaptive radiation therapy and the incorporation of functional information into treatment. MRgRT can potentially transform radiation oncology by improving tumour control and quality of life after radiation therapy and increasing convenience of treatment by shortening treatment courses for patients. Multiple groups have developed clinical implementations of MRgRT predominantly in the abdomen and pelvis, with patients having been treated since 2014. While studies of MRgRT have primarily been dosimetric so far, an increasing number of trials are underway examining the potential clinical benefits of MRgRT, with coordinated efforts to rigorously evaluate the benefits of the promising technology. This review discusses the current implementations, studies, potential benefits and challenges of MRgRT.
Collapse
Affiliation(s)
- Stephen Chin
- Department of Clinical Oncology, The Christie NHS Foundation Trust, Manchester, UK
- Westmead Clinical School, University of Sydney, Sydney, New South Wales, Australia
| | - Cynthia L Eccles
- Department of Radiotherapy, The Christie NHS Foundation Trust, Manchester, UK
- Division of Cancer Sciences, The University of Manchester, Manchester, UK
| | - Alan McWilliam
- Division of Cancer Sciences, The University of Manchester, Manchester, UK
- Christie Medical Physics and Engineering, The Christie NHS Foundation Trust, Manchester, UK
| | - Robert Chuter
- Division of Cancer Sciences, The University of Manchester, Manchester, UK
- Christie Medical Physics and Engineering, The Christie NHS Foundation Trust, Manchester, UK
| | - Emma Walker
- Christie Medical Physics and Engineering, The Christie NHS Foundation Trust, Manchester, UK
| | - Philip Whitehurst
- Christie Medical Physics and Engineering, The Christie NHS Foundation Trust, Manchester, UK
| | - Joseph Berresford
- Christie Medical Physics and Engineering, The Christie NHS Foundation Trust, Manchester, UK
| | - Marcel Van Herk
- Division of Cancer Sciences, The University of Manchester, Manchester, UK
- Christie Medical Physics and Engineering, The Christie NHS Foundation Trust, Manchester, UK
| | - Peter J Hoskin
- Department of Clinical Oncology, The Christie NHS Foundation Trust, Manchester, UK
- Division of Cancer Sciences, The University of Manchester, Manchester, UK
| | - Ananya Choudhury
- Department of Clinical Oncology, The Christie NHS Foundation Trust, Manchester, UK
- Division of Cancer Sciences, The University of Manchester, Manchester, UK
| |
Collapse
|
|
27
|
Lee JE, Diederich CJ, Bok R, Sriram R, Santos RD, Noworolski SM, Salgaonkar VA, Adams MS, Vigneron DB, Kurhanewicz J. Assessing high-intensity focused ultrasound treatment of prostate cancer with hyperpolarized 13 C dual-agent imaging of metabolism and perfusion. NMR IN BIOMEDICINE 2019; 32:e3962. [PMID: 30022550 PMCID: PMC6338537 DOI: 10.1002/nbm.3962] [Citation(s) in RCA: 8] [Impact Index Per Article: 1.6] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Subscribe] [Scholar Register] [Received: 02/22/2018] [Revised: 05/17/2018] [Accepted: 05/19/2018] [Indexed: 05/05/2023]
Abstract
The goal of the study was to establish early hyperpolarized (HP) 13 C MRI metabolic and perfusion changes that predict effective high-intensity focused ultrasound (HIFU) ablation and lead to improved adjuvant treatment of partially treated regions. To accomplish this a combined HP dual-agent (13 C pyruvate and 13 C urea) 13 C MRI/multiparametric 1 H MRI approach was used to measure prostate cancer metabolism and perfusion 3-4 h, 1 d, and 5 d after exposure to ablative and sub-lethal doses of HIFU within adenocarcinoma of mouse prostate tumors using a focused ultrasound applicator designed for murine studies. Pathologic and immunohistochemical analysis of the ablated tumor demonstrated fragmented, non-viable cells and vasculature consistent with coagulative necrosis, and a mixture of destroyed tissue and highly proliferative, poorly differentiated tumor cells in tumor tissues exposed to sub-lethal heat doses in the ablative margin. In ablated regions, the intensity of HP 13 C lactate or HP 13 C urea and dynamic contrast-enhanced (DCE) MRI area under the curve images were reduced to the level of background noise by 3-4 h after treatment with no recovery by the 5 d time point in either case. In the tissues that received sub-lethal heat dose, there was a significant 60% ± 12.4% drop in HP 13 C lactate production and a significant 30 ± 13.7% drop in urea perfusion 3-4 h after treatment, followed by recovery to baseline by 5 d after treatment. DCE MRI Ktrans showed a similar trend to HP 13 C urea, demonstrating a complete loss of perfusion with no recovery in the ablated region, while having a 40%-50% decrease 3-4 h after treatment followed by recovery to baseline values by 5 d in the margin region. The utility of the HP 13 C MR measures of perfusion and metabolism in optimizing focal HIFU, either alone or in combination with adjuvant therapy, deserves further testing in future studies.
Collapse
Affiliation(s)
- Jessie E. Lee
- Department of Radiology and Biomedical Imaging, University of California, San Francisco
- University of California, Berkeley, and University of California, San Francisco Joint Graduate Program in Bioengineering
| | - Chris J. Diederich
- Department of Radiology and Biomedical Imaging, University of California, San Francisco
- University of California, Berkeley, and University of California, San Francisco Joint Graduate Program in Bioengineering
- Department of Radiation Oncology, University of California, San Francisco
| | - Robert Bok
- Department of Radiology and Biomedical Imaging, University of California, San Francisco
| | - Renuka Sriram
- Department of Radiology and Biomedical Imaging, University of California, San Francisco
| | - Romelyn Delos Santos
- Department of Radiology and Biomedical Imaging, University of California, San Francisco
| | - Susan M. Noworolski
- Department of Radiology and Biomedical Imaging, University of California, San Francisco
- University of California, Berkeley, and University of California, San Francisco Joint Graduate Program in Bioengineering
| | | | - Matthew S. Adams
- University of California, Berkeley, and University of California, San Francisco Joint Graduate Program in Bioengineering
- Department of Radiation Oncology, University of California, San Francisco
| | - Daniel B. Vigneron
- Department of Radiology and Biomedical Imaging, University of California, San Francisco
- University of California, Berkeley, and University of California, San Francisco Joint Graduate Program in Bioengineering
| | - John Kurhanewicz
- Department of Radiology and Biomedical Imaging, University of California, San Francisco
- University of California, Berkeley, and University of California, San Francisco Joint Graduate Program in Bioengineering
| |
Collapse
|
|
28
|
MRI basics for radiation oncologists. Clin Transl Radiat Oncol 2019; 18:74-79. [PMID: 31341980 PMCID: PMC6630156 DOI: 10.1016/j.ctro.2019.04.008] [Citation(s) in RCA: 15] [Impact Index Per Article: 3.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/22/2019] [Revised: 04/09/2019] [Accepted: 04/09/2019] [Indexed: 02/01/2023] Open
Abstract
Issues of MRI that are relevant for radiation oncologists are addressed. Radiation oncology requires dedicated scan protocols. Use of diagnostic protocols is not recommended for radiotherapy. MR images must be made in treatment position with the standard positioning devices. Safety screening prior to entering the MRI room is crucial.
MRI is increasingly used in radiation oncology to facilitate tumor and organ-at-risk delineation and image guidance. In this review, we address issues of MRI that are relevant for radiation oncologists when interpreting MR images offered for radiotherapy. Whether MRI is used in combination with CT or in an MRI-only workflow, it is generally necessary to ensure that MR images are acquired in treatment position, using the positioning and fixation devices that are commonly applied in radiotherapy. For target delineation, often a series of separate image sets are used with distinct image contrasts, acquired within a single exam. MR images can suffer from image distortions. While this can be avoided with dedicated scan protocols, in a diagnostic setting geometrical fidelity is less relevant and is therefore less accounted for. Since geometrical fidelity is of utmost importance in radiation oncology, it requires dedicated scan protocols. The strong magnetic field of an MRI scanner and the use of radiofrequency radiation can cause safety hazards if not properly addressed. Safety screening is crucial for every patient and every operator prior to entering the MRI room.
Collapse
|
|
29
|
Chen W, Zhang H, Zeng X, Chen L, Fang G, Cai H, Zhong X. Phase‐shifted pentafluorobutane nanoparticles for ultrasound imaging and ultrasound‐mediated hypoxia modulation. J Cell Biochem 2019; 120:16543-16552. [PMID: 31099025 DOI: 10.1002/jcb.28914] [Citation(s) in RCA: 5] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/08/2018] [Revised: 03/18/2019] [Accepted: 04/05/2019] [Indexed: 12/18/2022]
Affiliation(s)
- Wei‐Jian Chen
- Department of Ultrasonography The First Affiliated Hospital of Jinan University Guangzhou China
| | - Hua Zhang
- The First Affiliated Hospital, Biomedical Translation Research Institute and School of Pharmacy Jinan University Guangzhou China
| | - Xue‐Yi Zeng
- Department of Chemistry, College of Chemistry and Materials Science Jinan University Guangzhou China
| | - Long Chen
- Department of Ultrasonography, Xiangyang Central Hospital Affiliated Hospital of Hubei University of Arts and Science Xiangyang China
| | - Gui‐Ting Fang
- Department of Ultrasonography The First Affiliated Hospital of Jinan University Guangzhou China
| | - Huai‐Hong Cai
- Department of Chemistry, College of Chemistry and Materials Science Jinan University Guangzhou China
| | - Xing Zhong
- Department of Ultrasonography The First Affiliated Hospital of Jinan University Guangzhou China
| |
Collapse
|
|
30
|
Li Y, Huang P, Peng H, Yue H, Wu M, Liu S, Qin R, Fan J, Han Y. Antitumor effects of Endostar(rh-endostatin) combined with gemcitabine in different administration sequences to treat Lewis lung carcinoma. Cancer Manag Res 2019; 11:3469-3479. [PMID: 31114380 PMCID: PMC6497885 DOI: 10.2147/cmar.s192868] [Citation(s) in RCA: 8] [Impact Index Per Article: 1.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/31/2018] [Accepted: 03/14/2019] [Indexed: 01/12/2023] Open
Abstract
Background: Endostatin therapy is known to efficiently inhibit angiogenesis and growth of endothelial cells. Nonetheless, the antitumor mechanisms of endostatin combined with chemotherapy remain to be elucidated. Methods: In our study, a Lewis lung carcinoma transplant mouse model was established and treated with the recombinant human [rh]-endostatin, Endostar, combined with gemcitabine at different sequences. 18F-FDG PET/CT imaging was performed to monitor tumor growth, and hypoxia was examined using an oxygen microelectrode. Vascular endothelial growth factor (VEGF) and alpha smooth muscle actin (α-SMA) levels were detected via immunohistochemistry analysis and cell cycle distributions were analyzed by flow cytometry. Results: Endostar decreased VEGF expression, improved hypoxia, and influenced cell cycle distributions. Simultaneous treatment of Endostar and gemcitabine displayed significantly tumor inhibition, possessed the lowest uptake of FDG, improved oxygen partial pressure, decreased expression of VEGF, and increased pericyte coverage. Cell cycle analysis demonstrated that cells accumulated in the S phase following gemcitabine treatment and G0/G1 arrest occurred following Endostar treatment. An increase of cells in G0/G1 phase was observed following treatment with Endostar and gemcitabine. Conclusions: Our study suggests that the combination therapy of Endostar with gemcitabine simutaneously may optimally enhance their individual antitumor effects.
Collapse
Affiliation(s)
- Yuan Li
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Pan Huang
- Neurology Department, Deyang People's Hospital, Deyang, Sichuan 618000, People's Republic of China
| | - Hongju Peng
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Hongcheng Yue
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Min Wu
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Shanshan Liu
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Rongsheng Qin
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Juan Fan
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| | - Yunwei Han
- The Oncology Department, Affiliated Hospital of Southwest Medical University, Lu Zhou, Si Chuan 646000, People's Republic of China
| |
Collapse
|
|
31
|
Naz S, Kishimoto S, Mitchell JB, Krishna MC. Imaging Metabolic Processes to Predict Radiation Responses. Semin Radiat Oncol 2019; 29:81-89. [PMID: 30573188 DOI: 10.1016/j.semradonc.2018.10.004] [Citation(s) in RCA: 2] [Impact Index Per Article: 0.4] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 12/25/2022]
Abstract
The aberrant vasculature in the tumor microenvironment creates hypoxic zones, poor perfusion, and high interstitial fluid pressure. Also, the tumor cell metabolic phenotype utilizes the aerobic glycolytic pathways for energy source and generation of cell mass. These physiologic and metabolic phenotypes in solid tumors are amenable for molecular imaging techniques to extract imaging biomarkers such as pO2 and enzyme kinetics reflecting glycolysis. The imaging biomarkers have value in diagnostic and prognostic purposes. Additionally, they can be used to guide choices for tailored treatment regimens. Electron paramagnetic resonance imaging for pO2 imaging and 13C magnetic resonance imaging with hyperpolarized 13C probes such as 13C-labeled pyruvate have shown significant potential in characterizing the tumor microenvironment physiologically and metabolically.
Collapse
Affiliation(s)
- Sarwat Naz
- Radiation Biology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD.
| | - Shun Kishimoto
- Radiation Biology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD
| | - James B Mitchell
- Radiation Biology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD
| | - Murali C Krishna
- Radiation Biology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, MD
| |
Collapse
|
|
32
|
The Effect of Neoadjuvant Androgen Deprivation Therapy on Tumor Hypoxia in High-Grade Prostate Cancer: An 18F-MISO PET-MRI Study. Int J Radiat Oncol Biol Phys 2018; 102:1210-1218. [DOI: 10.1016/j.ijrobp.2018.02.170] [Citation(s) in RCA: 9] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/23/2017] [Revised: 02/16/2018] [Accepted: 02/28/2018] [Indexed: 12/16/2022]
|
|
33
|
Wang H, Chao Y, Liu J, Zhu W, Wang G, Xu L, Liu Z. Photosensitizer-crosslinked in-situ polymerization on catalase for tumor hypoxia modulation & enhanced photodynamic therapy. Biomaterials 2018; 181:310-317. [DOI: 10.1016/j.biomaterials.2018.08.011] [Citation(s) in RCA: 126] [Impact Index Per Article: 21.0] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 02/26/2018] [Revised: 08/03/2018] [Accepted: 08/03/2018] [Indexed: 01/15/2023]
|
|
34
|
Desmet CM, Tran LBA, Danhier P, Gallez B. Characterization of a clinically used charcoal suspension for in vivo EPR oximetry. MAGNETIC RESONANCE MATERIALS IN PHYSICS BIOLOGY AND MEDICINE 2018; 32:205-212. [DOI: 10.1007/s10334-018-0704-x] [Citation(s) in RCA: 4] [Impact Index Per Article: 0.7] [Reference Citation Analysis] [Track Full Text] [Subscribe] [Scholar Register] [Received: 05/30/2018] [Revised: 08/21/2018] [Accepted: 08/31/2018] [Indexed: 12/18/2022]
|
|
35
|
Dynamic Contrast-Enhanced Magnetic Resonance Imaging of Advanced Cervical Carcinoma: The Advantage of Perfusion Parameters From the Peripheral Region in Predicting the Early Response to Radiotherapy. Int J Gynecol Cancer 2018; 28:1342-1349. [DOI: 10.1097/igc.0000000000001308] [Citation(s) in RCA: 4] [Impact Index Per Article: 0.7] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 11/25/2022] Open
Abstract
ObjectiveThis study aimed to investigate the importance of perfusion parameters from the peripheral region in predicting the early response to radiotherapy for advanced cervical carcinoma by using dynamic contrast-enhanced (DCE) perfusion magnetic resonance imaging (MRI).MethodsOne hundred eight patients with advanced cervical carcinoma were enrolled into this study. Dynamic contrast-enhanced perfusion MR examinations were performed for all the patients before radiotherapy. Perfusion parameters were obtained from the central region and the peripheral region of tumor respectively. After radiotherapy, the patients were classified into responders and nonresponders according to tumor shrinkage on the basis of follow-up MRI examination. The mean follow-up time lasted 12 months. The perfusion parameters were compared between the 2 groups. The relationship between perfusion parameters from 2 different regions of tumor and treatment effect was analyzed.ResultsThe mean value of volume transfer constant (Ktrans), rate constant (Kep) or extravascular extracellular volume fraction (Ve) from the peripheral region was higher than that from the central region of tumor, respectively (P = 0.01, 004, 0.03). Responders had higher Ktransperipheral (Ktrans from the peripheral region) and Ktranscentral (Ktrans from the central region) values than nonresponders (P = 0.04, 0.01). Responders had higher Kepperipheral (Kep from the peripheral region) than nonresponders (P = 0.03). Responders had lower Veperipheral (Ve from the peripheral region) than nonresponders (P = 0.04). At logistic regression analysis, the perfusion parameters that had predicting value were Ktransperipheral, Veperipheral, Kepperipheral and Ktranscentral according to diagnostic potency.ConclusionsCompared with perfusion parameters from the central region of tumor, perfusion parameters from the peripheral region are more valuable in predicting the early response to radiotherapy for advanced cervical carcinoma.
Collapse
|
|
36
|
He Y, Zhu Q, Chen M, Huang Q, Wang W, Li Q, Huang Y, Di W. The changing 50% inhibitory concentration (IC50) of cisplatin: a pilot study on the artifacts of the MTT assay and the precise measurement of density-dependent chemoresistance in ovarian cancer. Oncotarget 2018; 7:70803-70821. [PMID: 27683123 PMCID: PMC5342590 DOI: 10.18632/oncotarget.12223] [Citation(s) in RCA: 55] [Impact Index Per Article: 9.2] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 04/28/2016] [Accepted: 09/14/2016] [Indexed: 01/22/2023] Open
Abstract
Inconsistencies in the half-maximal (50%) inhibitory concentration (IC50) data for anticancer chemotherapeutic agents have yielded irreproducible experimental results and thus reciprocally contradictory theories in modern cancer research. The MTT assay is currently the most extensively used method for IC50 measurements. Here, we dissected the critical reasons behind MTT-dependent IC50 inconsistencies. We showed that IC50 errors caused by the technical deficiencies of the MTT assay are large and not adjustable (range: 300-11,000%). To overcome severe MTT artifacts, we developed an unbiased direct IC50 measurement method, the limiting dilution assay. This detection technique led us to the discovery of the inherent density-dependent chemoresistance variation of cancer cells, which is manifold and unpredictable in its forms. The subsequent intracellular signaling pathway analysis indicated that pAkt and p62 expression levels correlated with alterations in the IC50 values for cisplatin in ovarian cancer, providing an explainable mechanism for this property. An in situ pAkt-and-p62-based immunohistochemical (IHCpAkt+p62) scoring system was thereby established. Both the limiting dilution assay and the IHCpAkt+p62 scoring system accurately predicted the primary chemoresistance against cisplatin in ovarian cancer patients. Furthermore, two distinct chemoresistant recurrence patterns were uncovered using these novel detection tools, which were linked to two different forms of density-chemoresistance relationships (positively vs. negatively correlated), respectively. An interpretation was given based on the cancer evolution theory. We concluded that the density-related IC50 uncertainty is a natural property of the cancer cells and that the precise measurement of the density-dependent IC50 spectrum can benefit both basic and clinical cancer research fields.
Collapse
Affiliation(s)
- Yifeng He
- Department of Obstetrics and Gynecology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.,Shanghai Key Laboratory of Gynecologic Oncology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.,Tumor Microenvironment and Metastasis Program, The Wistar Institute, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA
| | - Qiujing Zhu
- Department of Obstetrics and Gynecology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.,Shanghai Key Laboratory of Gynecologic Oncology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.,State Key Laboratory of Oncogene and Related Genes, Shanghai Cancer Institute, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
| | - Mo Chen
- Department of Gynecology, Obstetrics and Gynecology Hospital, Fudan University, Shanghai 200011, China
| | - Qihong Huang
- Tumor Microenvironment and Metastasis Program, The Wistar Institute, University of Pennsylvania, Philadelphia, Pennsylvania 19104, USA
| | - Wenjing Wang
- Department of Obstetrics and Gynecology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.,Shanghai Key Laboratory of Gynecologic Oncology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.,State Key Laboratory of Oncogene and Related Genes, Shanghai Cancer Institute, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
| | - Qing Li
- Department of Obstetrics and Gynecology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.,Shanghai Key Laboratory of Gynecologic Oncology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.,State Key Laboratory of Oncogene and Related Genes, Shanghai Cancer Institute, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
| | - Yuting Huang
- Children's Research Institute, Children's National Medical Center, Washington DC 20010, USA
| | - Wen Di
- Department of Obstetrics and Gynecology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.,Shanghai Key Laboratory of Gynecologic Oncology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China.,State Key Laboratory of Oncogene and Related Genes, Shanghai Cancer Institute, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
| |
Collapse
|
|
37
|
Zhou H, Arias-Ramos N, López-Larrubia P, Mason RP, Cerdán S, Pacheco-Torres J. Oxygenation Imaging by Nuclear Magnetic Resonance Methods. Methods Mol Biol 2018; 1718:297-313. [PMID: 29341016 DOI: 10.1007/978-1-4939-7531-0_18] [Citation(s) in RCA: 3] [Impact Index Per Article: 0.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 12/12/2022]
Abstract
Oxygen monitoring is a topic of exhaustive research due to its central role in many biological processes, from energy metabolism to gene regulation. The ability to monitor in vivo the physiological distribution and the dynamics of oxygen from subcellular to macroscopic levels is a prerequisite to better understand the mechanisms associated with both normal and disease states (cancer, neurodegeneration, stroke, etc.). This chapter focuses on magnetic resonance imaging (MRI) based techniques to assess oxygenation in vivo. The first methodology uses injected fluorinated agents to provide quantitative pO2 measurements with high precision and suitable spatial and temporal resolution for many applications. The second method exploits changes in endogenous contrasts, i.e., deoxyhemoglobin and oxygen molecules through measurements of T 2* and T 1, in response to an intervention to qualitatively evaluate hypoxia and its potential modulation.
Collapse
Affiliation(s)
- Heling Zhou
- Prognostic Imaging Research Laboratory, Department of Radiology, UT Southwestern Medical Center, Dallas, TX, USA
| | - Nuria Arias-Ramos
- Departament de Bioquímica i Biologia Molecular, Unitat de Bioquímica de Biociències, Edifici Cs, Universitat Autònoma de Barcelona, Cerdanyola del Vallès, Spain
| | - Pilar López-Larrubia
- Instituto de Investigaciones Biomédicas 'Alberto Sols' C.S.I.C./U.A.M., Madrid, Spain
| | - Ralph P Mason
- Prognostic Imaging Research Laboratory, Department of Radiology, UT Southwestern Medical Center, Dallas, TX, USA
| | - Sebastián Cerdán
- Instituto de Investigaciones Biomédicas 'Alberto Sols' C.S.I.C./U.A.M., Madrid, Spain
| | - Jesús Pacheco-Torres
- Instituto de Neurociencias, Consejo Superior de Investigaciones Científicas, Universidad Miguel Hernández, San Juan de Alicante, Alicante, Spain.
| |
Collapse
|
|
38
|
Peerlings J, Van De Voorde L, Mitea C, Larue R, Yaromina A, Sandeleanu S, Spiegelberg L, Dubois L, Lambin P, Mottaghy FM. Hypoxia and hypoxia response-associated molecular markers in esophageal cancer: A systematic review. Methods 2017; 130:51-62. [PMID: 28705470 DOI: 10.1016/j.ymeth.2017.07.002] [Citation(s) in RCA: 38] [Impact Index Per Article: 5.4] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 05/01/2017] [Revised: 06/08/2017] [Accepted: 07/04/2017] [Indexed: 12/22/2022] Open
Abstract
PURPOSE In this systematic review, the existing evidence of available hypoxia-associated molecular response biomarkers in esophageal cancer (EC) patients is summarized and set into the context of the role of hypoxia in the prediction of esophageal cancer, treatment response and treatment outcome. METHODS A systematic literature search was performed in Web of Science, MEDLINE, and PubMed databases using the keywords: hypoxia, esophagus, cancer, treatment outcome and treatment response. Eligible publications were independently evaluated by two reviewers. In total, 22 out of 419 records were included for systematic review. The described search strategy was applied weekly, with the last update being performed on April 3rd, 2017. RESULTS In esophageal cancer, several (non-)invasive biomarkers for hypoxia could be identified. Independent prognostic factors for treatment response include HIF-1α, CA IX, GLUT-1 overexpression and elevated uptake of the PET-tracer 18F-fluoroerythronitroimidazole (18F-FETNIM). Hypoxia-associated molecular responses represents a clinically relevant phenomenon in esophageal cancer and detection of elevated levels of hypoxia-associated biomarkers and tends to be associated with poor treatment outcome (i.e., overall survival, disease-free survival, complete response and local control). CONCLUSION Evaluation of tumor micro-environmental conditions, such as intratumoral hypoxia, is important to predict treatment outcome and efficacy. Promising non-invasive imaging-techniques have been suggested to assess tumor hypoxia and hypoxia-associated molecular responses. However, extensive validation in EC is lacking. Hypoxia-associated markers that are independent prognostic factors could potentially provide targets for novel treatment strategies to improve treatment outcome. For personalized hypoxia-guided treatment, safe and reliable makers for tumor hypoxia are needed to select suitable patients.
Collapse
Affiliation(s)
- Jurgen Peerlings
- MAASTRO Clinic, Department of Radiation Oncology, GROW - School for Oncology and Developmental Biology, Maastricht University Medical Centre+, Maastricht, The Netherlands; Department of Radiology and Nuclear Medicine, Maastricht University Medical Centre+, Maastricht, The Netherlands.
| | - Lien Van De Voorde
- MAASTRO Clinic, Department of Radiation Oncology, GROW - School for Oncology and Developmental Biology, Maastricht University Medical Centre+, Maastricht, The Netherlands
| | - Cristina Mitea
- Department of Radiology and Nuclear Medicine, Maastricht University Medical Centre+, Maastricht, The Netherlands
| | - Ruben Larue
- MAASTRO Clinic, Department of Radiation Oncology, GROW - School for Oncology and Developmental Biology, Maastricht University Medical Centre+, Maastricht, The Netherlands
| | - Ala Yaromina
- MAASTRO Clinic, Department of Radiation Oncology, GROW - School for Oncology and Developmental Biology, Maastricht University Medical Centre+, Maastricht, The Netherlands
| | - Sebastian Sandeleanu
- MAASTRO Clinic, Department of Radiation Oncology, GROW - School for Oncology and Developmental Biology, Maastricht University Medical Centre+, Maastricht, The Netherlands
| | - Linda Spiegelberg
- MAASTRO Clinic, Department of Radiation Oncology, GROW - School for Oncology and Developmental Biology, Maastricht University Medical Centre+, Maastricht, The Netherlands
| | - Ludwig Dubois
- MAASTRO Clinic, Department of Radiation Oncology, GROW - School for Oncology and Developmental Biology, Maastricht University Medical Centre+, Maastricht, The Netherlands
| | - Philippe Lambin
- MAASTRO Clinic, Department of Radiation Oncology, GROW - School for Oncology and Developmental Biology, Maastricht University Medical Centre+, Maastricht, The Netherlands
| | - Felix M Mottaghy
- Department of Radiology and Nuclear Medicine, Maastricht University Medical Centre+, Maastricht, The Netherlands; Department of Nuclear Medicine, University Hospital RWTH Aachen University, Aachen, Germany
| |
Collapse
|
|
39
|
Chen GZ, Zhu HC, Dai WS, Zeng XN, Luo JH, Sun XC. The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity. J Thorac Dis 2017; 9:849-859. [PMID: 28449496 PMCID: PMC5394057 DOI: 10.21037/jtd.2017.03.23] [Citation(s) in RCA: 56] [Impact Index Per Article: 8.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/07/2016] [Accepted: 01/19/2017] [Indexed: 12/21/2022]
Abstract
Esophageal cancer is the eighth most common cancer and the sixth leading cause of cancer-related death worldwide. Surgery is the primary form of treatment, but the survival is poor, especially for patients with locally advanced esophageal cancer. Radiotherapy has been a critical treatment option that may be combined with chemotherapy in patients with unresectable esophageal cancer. However, resistance to chemoradiotherapy might result in treatment failures and cancer relapse. This review will mainly focus on the possible cellular mechanisms and tumor-associated microenvironmental (TAM) factors that result in radioresistance in patients with esophageal cancer. In addition, current strategies to increase radiosensitivity, including targeted therapy and the use of radiosensitive biomarkers in clinical treatment, are discussed in this review.
Collapse
Affiliation(s)
- Guang-Zong Chen
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Hong-Cheng Zhu
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Wang-Shu Dai
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Xiao-Ning Zeng
- Department of Respiratory Medicine, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Jin-Hua Luo
- Department of Thoracic Surgery, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Xin-Chen Sun
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| |
Collapse
|
|
40
|
Swartz HM. Using Stable Free Radicals to Obtain Unique and Clinically Useful Data In Vivo in Human Subjects. RADIATION PROTECTION DOSIMETRY 2016; 172:3-15. [PMID: 27886997 PMCID: PMC6061194 DOI: 10.1093/rpd/ncw323] [Citation(s) in RCA: 4] [Impact Index Per Article: 0.5] [Reference Citation Analysis] [Abstract] [MESH Headings] [Track Full Text] [Subscribe] [Scholar Register] [Accepted: 10/30/2016] [Indexed: 06/06/2023]
Abstract
This paper attempts to: (1) provide a critical overview of the challenges and opportunities to extend electron paramagnetic resonance (EPR) into practical applications in human subjects, based on EPR measurements made in vivo; (2) summarize the clinical applications of EPR for improving treatments in cancer, wound healing and diabetic care, emphasizing EPR's unique capability to measure tissue oxygen repeatedly and with particular sensitivity to hypoxia and (3) summarize the capabilities of in vivo EPR to measure radiation dose for triage and medical guidance after a large-scale radiation exposure. The conclusion is that while still at a relatively early stage of its development and availability, clinical applications of EPR already have demonstrated significant value and the field is likely to grow in both the extent of its applications and its impact on significant problems.
Collapse
Affiliation(s)
- Harold M Swartz
- EPR Center for the Study of Viable Systems at Dartmouth, Department of Radiology, Geisel School of Medicine at Dartmouth, HB 7785 One Medical Center Drive, Lebanon, NH 03756, USA
- Division of Radiation Oncology, Department of Medicine, Geisel School of Medicine at Dartmouth, Lebanon, NH, USA
| |
Collapse
|
|
41
|
Grassberger C, Paganetti H. Methodologies in the modeling of combined chemo-radiation treatments. Phys Med Biol 2016; 61:R344-R367. [DOI: 10.1088/0031-9155/61/21/r344] [Citation(s) in RCA: 15] [Impact Index Per Article: 1.9] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 12/26/2022]
|
|
42
|
Song X, Feng L, Liang C, Yang K, Liu Z. Ultrasound Triggered Tumor Oxygenation with Oxygen-Shuttle Nanoperfluorocarbon to Overcome Hypoxia-Associated Resistance in Cancer Therapies. NANO LETTERS 2016; 16:6145-6153. [PMID: 27622835 DOI: 10.1021/acs.nanolett.6b02365] [Citation(s) in RCA: 425] [Impact Index Per Article: 53.1] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Subscribe] [Scholar Register] [Indexed: 05/16/2023]
Abstract
Tumor hypoxia is known to be one of critical reasons that limit the efficacy of cancer therapies, particularly photodynamic therapy (PDT) and radiotherapy (RT) in which oxygen is needed in the process of cancer cell destruction. Herein, taking advantages of the great biocompatibility and high oxygen dissolving ability of perfluorocarbon (PFC), we develop an innovative strategy to modulate the tumor hypoxic microenvironment using nano-PFC as an oxygen shuttle for ultrasound triggered tumor-specific delivery of oxygen. In our experiment, nanodroplets of PFC stabilized by albumin are intravenously injected into tumor-bearing mice under hyperoxic breathing. With a low-power clinically adapted ultrasound transducer applied on their tumor, PFC nanodroplets that adsorb oxygen in the lung would rapidly release oxygen in the tumor under ultrasound stimulation, and then circulate back into the lung for reoxygenation. Such repeated cycles would result in dramatically enhanced tumor oxygenation and thus remarkably improved therapeutic outcomes in both PDT and RT treatment of tumors. Importantly, our strategy may be applied for different types of tumor models. Hence, this work presents a simple strategy to promote tumor oxygenation with great efficiency using agents and instruments readily available in the clinic, so as to overcome the hypoxia-associated resistance in cancer treatment.
Collapse
Affiliation(s)
- Xuejiao Song
- Institute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University , Suzhou 215123, China
| | - Liangzhu Feng
- Institute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University , Suzhou 215123, China
| | - Chao Liang
- Institute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University , Suzhou 215123, China
| | - Kai Yang
- School of Radiation Medicine and Protection & School for Radiological and Interdisciplinary Sciences (RAD-X), Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions Medical College of Soochow University , Suzhou, Jiangsu 21513, China
| | - Zhuang Liu
- Institute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University , Suzhou 215123, China
| |
Collapse
|
|
43
|
Composition and Function of the Interstitial Fluid. Protein Sci 2016. [DOI: 10.1201/9781315374307-2] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 11/11/2022]
|
|
44
|
Oike T, Sato H, Noda SE, Nakano T. Translational Research to Improve the Efficacy of Carbon Ion Radiotherapy: Experience of Gunma University. Front Oncol 2016; 6:139. [PMID: 27376029 PMCID: PMC4899433 DOI: 10.3389/fonc.2016.00139] [Citation(s) in RCA: 16] [Impact Index Per Article: 2.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/27/2016] [Accepted: 05/23/2016] [Indexed: 11/13/2022] Open
Abstract
Carbon ion radiotherapy holds great promise for cancer therapy. Clinical data show that carbon ion radiotherapy is an effective treatment for tumors that are resistant to X-ray radiotherapy. Since 1994 in Japan, the National Institute of Radiological Sciences has been heading the development of carbon ion radiotherapy using the Heavy Ion Medical Accelerator in Chiba. The Gunma University Heavy Ion Medical Center (GHMC) was established in the year 2006 as a proof-of-principle institute for carbon ion radiotherapy with a view to facilitating the worldwide spread of compact accelerator systems. Along with the management of more than 1900 cancer patients to date, GHMC engages in translational research to improve the treatment efficacy of carbon ion radiotherapy. Research aimed at guiding patient selection is of utmost importance for making the most of carbon ion radiotherapy, which is an extremely limited medical resource. Intratumoral oxygen levels, radiation-induced cellular apoptosis, the capacity to repair DNA double-strand breaks, and the mutational status of tumor protein p53 and epidermal growth factor receptor genes are all associated with X-ray sensitivity. Assays for these factors are useful in the identification of X-ray-resistant tumors for which carbon ion radiotherapy would be beneficial. Research aimed at optimizing treatments based on carbon ion radiotherapy is also important. This includes assessment of dose fractionation, normal tissue toxicity, tumor cell motility, and bystander effects. Furthermore, the efficacy of carbon ion radiotherapy will likely be enhanced by research into combined treatment with other modalities such as chemotherapy. Several clinically available chemotherapeutic drugs (carboplatin, paclitaxel, and etoposide) and drugs at the developmental stage (Wee-1 and heat shock protein 90 inhibitors) show a sensitizing effect on tumor cells treated with carbon ions. Additionally, the efficacy of carbon ion radiotherapy can be improved by combining it with cancer immunotherapy. Clinical validation of preclinical findings is necessary to further improve the treatment efficacy of carbon ion radiotherapy.
Collapse
Affiliation(s)
- Takahiro Oike
- Department of Radiation Oncology, Gunma University Graduate School of Medicine , Gunma , Japan
| | - Hiro Sato
- Department of Radiation Oncology, Gunma University Graduate School of Medicine , Gunma , Japan
| | - Shin-Ei Noda
- Department of Radiation Oncology, Gunma University Graduate School of Medicine , Gunma , Japan
| | - Takashi Nakano
- Department of Radiation Oncology, Gunma University Graduate School of Medicine, Gunma, Japan; Gunma University Heavy Ion Medical Center, Gunma, Japan
| |
Collapse
|
|
45
|
Baumann R, Depping R, Delaperriere M, Dunst J. Targeting hypoxia to overcome radiation resistance in head & neck cancers: real challenge or clinical fairytale? Expert Rev Anticancer Ther 2016; 16:751-8. [PMID: 27253509 DOI: 10.1080/14737140.2016.1192467] [Citation(s) in RCA: 33] [Impact Index Per Article: 4.1] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 01/11/2023]
Abstract
INTRODUCTION Tumor hypoxia is a major cause for failure of therapy in patients with inoperable head and neck cancers. AREAS COVERED Various anti-hypoxic treatment strategies (e.g. hyperbaric oxygenation, hypoxic cell sensitizers) have been tested in clinical trials in head and neck cancer over the past 30 years and have shown modest improvements in combination with radiotherapy in meta-analyses. Anemia worsens tumor hypoxia, but anemia correction had no significant effect. New approaches (e.g. anti-HIF-directed molecular therapies) have just entered early clinical studies and data are lacking. Expert commentary: A new attractive and promising approach derives from recent advances in imaging and radiotherapy delivery. Progress in imaging of hypoxia (e.g. by positron emission tomography) can select patients for specific therapies and may, in particular, facilitate anti-hypoxia-directed radiotherapy which has become feasible with advanced radiotherapy techniques (IMRT with 'dose-painting'). The combination of both methods may offer a powerful tool for effective targeting of hypoxia in the near future.
Collapse
Affiliation(s)
- René Baumann
- a Department of Radiation Oncology , Christian-Albrechts-University Kiel , Kiel , Germany
| | - Reinhard Depping
- b Institute of Physiology , University of Luebeck , Luebeck , Germany
| | - Marc Delaperriere
- a Department of Radiation Oncology , Christian-Albrechts-University Kiel , Kiel , Germany
| | - Juergen Dunst
- a Department of Radiation Oncology , Christian-Albrechts-University Kiel , Kiel , Germany
| |
Collapse
|
|
46
|
Abstract
The tumor microenvironment (TME) is a complex, heterogeneous, and dominant component of solid tumors. Cancer imaging strategies of a subset of characteristics of the TME are under active development, and currently used modalities and novel approaches are summarized in this article. Understanding the dynamic and evolving functions of the TME is critical to accurately inform imaging and clinical care of cancer. Novel insights into distinct roles of the TME in cancer progression urge careful interpretation of imaging data and impel the development of novel modalities.
Collapse
Affiliation(s)
- Valerie S LeBleu
- From the Department of Cancer Biology, Metastasis Research Center, University of Texas MD Anderson Cancer Center, Houston, TX
| |
Collapse
|
|
47
|
Sandulache VC, Chen Y, Skinner HD, Lu T, Feng L, Court LE, Myers JN, Meyn RE, Fuller CD, Bankson JA, Lai SY. Acute Tumor Lactate Perturbations as a Biomarker of Genotoxic Stress: Development of a Biochemical Model. Mol Cancer Ther 2015; 14:2901-8. [PMID: 26376962 DOI: 10.1158/1535-7163.mct-15-0217] [Citation(s) in RCA: 12] [Impact Index Per Article: 1.3] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/16/2015] [Accepted: 09/07/2015] [Indexed: 12/15/2022]
Abstract
Ionizing radiation is the primary nonsurgical treatment modality for solid tumors. Its effectiveness is impacted by temporal constraints such as fractionation, hypoxia, and development of radioresistant clones. Biomarkers of acute radiation response are essential to developing more effective clinical algorithms. We hypothesized that acute perturbations in tumor lactate levels act as a surrogate marker of radiation response. In vitro experiments were carried out using validated human-derived cell lines from three histologies: anaplastic thyroid carcinoma (ATC), head and neck squamous cell carcinoma (HNSCC), and papillary thyroid carcinoma (PTC). Cellular metabolic activity was measured using standard biochemical assays. In vivo validation was performed using both an orthotopic and a flank derivative of a previously established ATC xenograft murine model. Irradiation of cells and tumors triggered a rapid, dose-dependent, transient decrease in lactate levels that was reversed by free radical scavengers. Acute lactate perturbations following irradiation could identify hypoxic conditions and correlated with hypoxia-induced radioresistance. Mutant TP53 cells and cells in which p53 activity was abrogated (shRNA) demonstrated a blunted lactate response to irradiation, consistent with a radioresistant phenotype. Lactate measurements therefore rapidly detected both induced (i.e., hypoxia) and intrinsic (i.e., mutTP53-driven) radioresistance. We conclude that lactate is a quantitative biomarker of acute genotoxic stress, with a temporal resolution that can inform clinical decision making. Combined with the spatial resolution of newly developed metabolic imaging platforms, this biomarker could lead to the development of truly individualized treatment strategies.
Collapse
Affiliation(s)
- Vlad C Sandulache
- Bobby R. Alford Department of Otolaryngology-Head and Neck Surgery, Baylor College of Medicine, Houston, Texas. Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas
| | - Yunyun Chen
- Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas
| | - Heath D Skinner
- Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas
| | - Tongtong Lu
- Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas
| | - Lei Feng
- Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, Texas
| | - Laurence E Court
- Department of Radiation Physics, The University of Texas MD Anderson Cancer Center, Houston, Texas
| | - Jeffrey N Myers
- Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas
| | - Raymond E Meyn
- Department of Experimental Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas
| | - Clifton D Fuller
- Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas
| | - James A Bankson
- Department of Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, Texas
| | - Stephen Y Lai
- Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas. Department of Molecular and Cellular Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas.
| |
Collapse
|
|
48
|
Xiang D, Shigdar S, Qiao G, Wang T, Kouzani AZ, Zhou SF, Kong L, Li Y, Pu C, Duan W. Nucleic acid aptamer-guided cancer therapeutics and diagnostics: the next generation of cancer medicine. Am J Cancer Res 2015; 5:23-42. [PMID: 25553096 PMCID: PMC4265746 DOI: 10.7150/thno.10202] [Citation(s) in RCA: 153] [Impact Index Per Article: 17.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/28/2014] [Accepted: 09/01/2014] [Indexed: 12/29/2022] Open
Abstract
Conventional anticancer therapies, such as chemo- and/or radio-therapy are often unable to completely eradicate cancers due to abnormal tumor microenvironment, as well as increased drug/radiation resistance. More effective therapeutic strategies for overcoming these obstacles are urgently in demand. Aptamers, as chemical antibodies that bind to targets with high affinity and specificity, are a promising new and novel agent for both cancer diagnostic and therapeutic applications. Aptamer-based cancer cell targeting facilitates the development of active targeting in which aptamer-mediated drug delivery could provide promising anticancer outcomes. This review is to update the current progress of aptamer-based cancer diagnosis and aptamer-mediated active targeting for cancer therapy in vivo, exploring the potential of this novel form of targeted cancer therapy.
Collapse
|
|
49
|
Omidi Y, Barar J. Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. BIOIMPACTS : BI 2014; 4:55-67. [PMID: 25035848 PMCID: PMC4097973 DOI: 10.5681/bi.2014.021] [Citation(s) in RCA: 39] [Impact Index Per Article: 3.9] [Reference Citation Analysis] [Abstract] [Key Words] [Download PDF] [Figures] [Subscribe] [Scholar Register] [Received: 04/22/2014] [Revised: 05/07/2014] [Accepted: 06/01/2014] [Indexed: 12/19/2022]
Abstract
Introduction: The genesis of cancer appears to be a complex matter, which is not simply based upon few genetic abnormalities/alteration. In fact, irregular microvasculature and aberrant interstitium of solid tumors impose significant pathophysiologic barrier functions against cancer treatment modalities, hence novel strategies should holistically target bioelements of tumor microenvironment (TME). In this study, we provide some overview and insights on TME and important strategies used to control the impacts of such pathophysiologic barriers.
Methods: We reviewed all relevant literature for the impacts of tumor interstitium and microvasculature within the TME as well as the significance of the implemented strategies.
Results: While tumorigenesis initiation seems to be in close relation with an emergence of hypoxia and alterations in epigenetic/genetic materials, large panoplies of molecular events emerge as intricate networks during oncogenesis to form unique lenient TME in favor of tumor progression. Within such irregular interstitium, immune system displays defective surveillance functionalities against malignant cells. Solid tumors show multifacial traits with coadaptation and self-regulation potentials, which bestow profound resistance against the currently used conventional chemotherapy and immunotherapy agents that target solely one face of the disease.
Conclusion: The cancerous cells attain unique abilities to form its permissive microenvironment, wherein (a) extracellular pH is dysregulated towards acidification, (b) extracellular matrix (ECM) is deformed, (c) stromal cells are cooperative with cancer cells, (d) immune system mechanisms are defective, (e) non-integrated irregular microvasculature with pores (120-1200 nm) are formed, and (h) interstitial fluid pressure is high. All these phenomena are against cancer treatment modalities. As a result, to control such abnormal pathophysiologic traits, novel cancer therapy strategies need to be devised using multifunctional nanomedicines and theranostics.
Collapse
Affiliation(s)
- Yadollah Omidi
- Research Center for Pharmaceutical Nanotechnology, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
| | - Jaleh Barar
- Research Center for Pharmaceutical Nanotechnology, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
| |
Collapse
|